Abbreviations
- ACTH Adreno-corticotropic hormone
- CASR Calcium sensing receptor
- CT Computed tomography
- FBHH Familial benign hypocalciuric hypercalcemia
- FNAB Fine needle aspiration biopsy
- FU Flouro-uracil
- MEN Multiple endocrine neoplasia
- MRI Magnetic resonance imaging
- MTC Medullary thyroid cancer
- NIH National institutes of health
- PHPT Primary hyperparathyroidism
- PTH Parathyroid hormone
- RAI Radioactive iodine
- RAIU Radioactive iodine uptake
- RFA Radiofrequency ablation
- TACE Transarterial chemoembolization
- SPECT Single photon emission computed tomography
- TSH Thyroid stimulating hormone
- VIP Vasoactive intestinal peptide
- ZES Zollinger Ellison syndrome
Introduction
Many endocrine diseases are appropriately managed surgically. The details of clinical presentation, diagnosis, and medical management are discussed in other sections of this book. This chapter provides an overview of the principles involved in the surgical therapy for these conditions. The indications for surgical intervention, relevant procedures, and the benefits of these procedures are discussed.
The Thyroid Gland
The thyroid gland arises in the midline as an endoderm-derived pharyngeal diverticulum around the third week of gestation. The paired median thyroid anlages then descend from their origin at the base of the tongue (foramen cecum) and ultimately form a bilobed thyroid gland anterolateral to the trachea and larynx. The thyroid lobes are connected just below the cricoid cartilage by an isthmus. The connection to the foramen cecum—the thyroglossal duct—separates and is partially resorbed by the sixth week of gestation. Its distal remnant forms the pyramidal lobe. The calcitonin-producing C cells are neuroectodermal in origin, arise from the fourth branchial pouch, and are located in the superoposterior aspect of the gland.
A number of embryologic or developmental abnormalities of the thyroid have been described and are related to the absence or mutations of thyroid differentiation factors, including thyroid transcription factors 1 and 2 (TTF-1 and TTF-2) and transcription factor Pax 8. Thyroglossal duct cysts are usually found in the midline, just inferior to the hyoid bone. A lingual thyroid results from maldescent of the median thyroid anlage and is often accompanied by agenesis of other thyroid tissue. Rests of thyroid tissue may be found anywhere in the central compartment of the neck, including the anterior mediastinum. Tongues of thyroid tissue are often seen to extend off the lower thyroid poles, particularly in large goiters. In contrast to the above, thyroid tissue in the lateral neck lymph nodes (lateral aberrant thyroid) almost always represents metastatic thyroid cancer and is not a developmental abnormality.
The adult thyroid gland is reddish-brown in color and weighs approximately 20 g. The thyroid gland is supplied by paired superior and inferior thyroid arteries. The former arise from the external carotid artery and the latter from the thyrocervical trunk. A thyroid ima artery arises directly from the aorta or innominate artery in approximately 2% of individuals and enters the isthmus, replacing an absent inferior artery.
The thyroid is drained by three sets of veins: the superior, middle, and inferior thyroid veins. The first two drain into the internal jugular vein, whereas the last drains into the innominate veins. Both recurrent laryngeal nerves arise from their respective vagus nerves and enter the larynx at the level of the cricothyroid articulation, posterior to the cricothyroid muscle. The left recurrent laryngeal nerve recurs around the ligamentum arteriosum and ascends to the larynx in the tracheoesophageal groove. The right recurrent laryngeal nerve recurs around the subclavian artery and runs 1 to 2 cm lateral to the tracheoesophageal groove at the level of the clavicle and courses obliquely to the larynx. The superior laryngeal nerves also arise from corresponding vagus nerves and divide into internal and external branches. The former provides sensation to the larynx and the latter innervates the cricothyroid muscles. A description of parathyroid embryology and anatomy is presented in the next section.
Prior to discussing indications for thyroidectomy, it is prudent to clarify the definitions of various thyroid resections. A description of these terms is presented in Table 26–1. Of note, nodulectomies are rarely performed, and most surgeons agree that thyroid lobectomy constitutes a minimum resection.
| Procedure | Description |
|---|---|
| Nodulectomy or lumpectomy | Removal of lesion with minimal surrounding tissue |
| Partial thyroidectomy | Removal of lesion and larger rim of normal tissue |
| Subtotal thyroidectomy | Bilateral removal of >50% of each lobe and an isthmusectomy |
| Lobectomy or hemithyroidectomy | Complete removal of a lobe and isthmus |
| Near-total thyroidectomy | Complete removal of one lobe and isthmus and all but 1 g (1 cm) of the contralateral lobe (tissue near ligament of Berry) |
| Total thyroidectomy | Complete removal of both thyroid lobes, isthmus, and pyramidal lobe |
Indications for Surgery
Thyroglossal duct remnants may become symptomatic, forming cysts, abscesses, and fistulae. There is also a 1% risk of thyroid cancer development in thyroglossal duct cysts. Most are papillary carcinomas, but very rarely a squamous cell carcinoma may develop. Medullary thyroid cancers do not occur at this site.
Treatment of thyroglossal duct remnants consists of the Sistrunk procedure, which involves removal of the cyst and duct up to the foramen cecum. Because the duct may pass anterior to, posterior to, or through the hyoid bone, it is generally recommended that the midsection of this bone is also resected in order to reduce the risk of recurrence. Surgery may also be needed for enlarged lingual thyroid tissue causing symptoms such as choking, dysphagia, airway obstruction, and hemorrhage. Prior to resection, care must be taken to determine whether the patient has any other functioning thyroid tissue, usually via a thyroid scan or ultrasound.
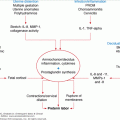
Hyperthyroidism may result from increased thyroid hormone production or release of stored thyroid hormone following injury to the thyroid gland such as that seen in subacute thyroiditis. This is an important distinction, because the former group of disorders leads to increased RAIU, whereas the latter are associated with low or normal RAIU. The most common causes of hyperthyroidism are diffuse toxic goiter (Graves disease), toxic multinodular goiter (Plummer disease), or a single toxic nodule, all of which cause increased RAIU. Rarer causes of hyperthyroidism with increased radioactive iodine uptake (RAIU) include a TSH–secreting tumor and a hydatidiform mole. Causes of hyperthyroidism without increased RAIU include subacute thyroiditis, excessive ingestion of medicinal thyroid hormone, struma ovarii, and thyroid hormone–secreting metastatic thyroid cancer. These latter conditions present with the usual symptoms and signs of hyperthyroidism but lack the extrathyroidal manifestations of Graves disease such as ophthalmopathy, pretibial myxedema, and thyroid acropachy.
Hyperthyroidism is characterized by a suppressed TSH in the presence of elevated free T4 levels. If free T4 is normal, free T3 levels should be measured because they are often elevated early in the course of disease. RAIU can be used to distinguish the various causes of hyperthyroidism. Approximately 90% of patients with Graves disease have elevated levels of thyroid-stimulating antibodies or immunoglobulins (thyroid-stimulating immunoglobulin).
Hyperthyroidism may be treated medically with antithyroid medications, but relapse is common when the medical treatment is discontinued. Ablation of the thyroid gland with RAI is the mainstay of treatment in North America for patients over 30 years of age. A dose of 8 to 12 mCi of I-131 taken orally is generally required. However, it is associated with a prolonged latency period before effective action, a slightly increased risk of future benign and malignant thyroid tumors, hyperparathyroidism, worsening ophthalmopathy (particularly in smokers), and unavoidable hypothyroidism (3% per year after the first year, independently of dosage). Furthermore, only about 50% of patients treated with RAI are euthyroid at 6 months. It is contraindicated in pregnant women, of concern in children, and should be avoided in women wishing to become pregnant for up to 1 year after treatment. Surgery overcomes many of the problems associated with RAI and achieves rapid control of hyperthyroidism with minimal side effects in experienced hands.
Absolute indications for thyroidectomy in patients with Graves disease include biopsy-proven suspicious or cancerous nodules, local compressive symptoms, severe reactions to antithyroid medications, reluctance to undergo RAI ablation, or fear of recurrence after RAI. Women who want to become pregnant after treatment or who develop side effects from antithyroid drugs during pregnancy are also candidates for thyroidectomy, as are children. Relative indications for thyroidectomy include patients in whom rapid control of the disease is desired; poorly compliant patients; and patients with severe ophthalmopathy, very large goiters, or low RAIU.
Patients are usually treated with antithyroid medications to render them euthyroid and to prevent the risk of thyroid storm. Propylthiouracil (100-200 mg 3 times daily) or methimazole (10-20 mg twice daily and then once daily) are the most commonly used medications and are continued up to the day of surgery. In patients who develop agranulocytosis as a complication of these medications, surgery should be deferred until granulocyte counts reach 1000 cells/uL. In addition, patients are also often treated with propranolol (10-40 mg 4 times daily) to control the adrenergic effects of hyperthyroidism. Relatively large doses may be necessary because of increased catabolism of the drug. Lugol solution (iodine and potassium iodide) or saturated solution of potassium iodide is also generally started about 10 days preoperatively to reduce the vascularity of the gland and decrease the risk of precipitating thyroid storm.
The extent of surgery is controversial and depends on multiple factors. For those patients who have experienced severe complications with antithyroid drugs, those who want to eliminate the risk of recurrence, and those with coexistent carcinoma or severe ophthalmopathy, total or near-total thyroidectomy is recommended. However, total thyroidectomy results in inevitable hypothyroidism and is potentially associated with higher complication rates when compared to lesser procedures. Thus, the procedure traditionally preferred by most surgeons for treating patients with Graves disease is a subtotal thyroidectomy. This may be accomplished safely by bilateral subtotal excision or by unilateral lobectomy and isthmusectomy with subtotal contralateral resection (Hartley-Dunhill procedure). Provided an adequate remnant is left behind, the procedure provides rapid relief of thyrotoxicosis while maintaining a euthyroid state without the need for thyroid hormone. However, it is difficult to define what constitutes an adequate remnant, although most surgeons consider a 4- to 7-g remnant sufficient in adults. Rates of postoperative hypothyroidism are variable and are primarily determined by remnant size, thyroid antibody titer, and whether hypothyroidism was reported to be subclinical or overt. Remnants <4 g are associated with a >50% risk of hypothyroidism, and those >8 g are associated with recurrence rates of 15%. Subtotal resections are associated with recurrence rates of 1.2% to 16.2%, and recurrence may take place many years after thyroidectomy. Therefore, many surgeons now consider avoidance of recurrence, rather than attainment of euthyroidism, as the goal for thyroidectomy in Graves disease. This is gradually leading to total or near-total thyroidectomy becoming the preferred operation for these patients. Patients who experience recurrence after surgery are usually treated with RAI. Patients with hyperthyroidism secondary to toxic multinodular goiter are managed similar to those with Graves disease. Subtotal thyroidectomy is generally advised, however, if no normal remnant can be left, a near-total or total thyroidectomy is recommended. Patients with a solitary toxic nodule >3 cm in size are best treated by ipsilateral lobectomy. Patients with smaller hot nodules may be managed by tumor enucleation or RAI ablation.
Thyroiditis, an inflammatory disorder of the thyroid gland, may be classified as acute, subacute, or chronic. Acute suppurative thyroiditis is diagnosed by fine-needle aspiration for cytology, smear, Gram stain, and culture and treated by incision and drainage and antibiotics. Acute thyroiditis is usually self-limited. Thyroidectomy, however, is occasionally needed for clinically coexistent suspicious nodules or cancer, local compressive symptoms, or persistent infection. Recurrent acute thyroiditis is often due to a persistent pyriform sinus fistula. Resection of the entire tract is necessary to prevent recurrence. Subacute and chronic thyroiditis is usually managed medically, but surgical resection is occasionally needed for local symptoms or relapse.
Goiters may be diffuse, uninodular, or multinodular. Thyroidectomy is indicated for goiters enlarging despite thyroxine suppression, those causing compressive symptoms (choking, dysphagia, hoarseness, dyspnea, or a positive Pemberton sign—dilation of neck veins and facial erythema on elevation of arms), nodules deemed suspicious by ultrasound, and goiters containing biopsy-proven suspicious or cancerous nodules. Goiters with a significant substernal component (>50%) are considered a relative indication for thyroidectomy. Subtotal thyroidectomy is the preferred treatment for multinodular goiters. A total lobectomy is performed on the side with the dominant nodule, and a subtotal thyroidectomy is performed on the other. A total or near-total thyroidectomy may be performed if there is no normal thyroid tissue present.
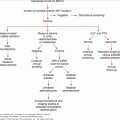
Approximately 4% of the North American population develops thyroid nodules. However, the incidence of clinical thyroid cancer is much lower (about 40 patients per million), thus making it imperative to determine which nodules require treatment with thyroidectomy. A thyroid nodule is more likely to be malignant if the patient has a history of therapeutic radiation to the head and neck (6.5-3000 cGy to the thyroid), a family history of thyroid cancer, Cowden syndrome, or MEN 2, and a personal history of thyroid cancer. Other features suggesting cancer include male sex, young (<20) or old (>70) age; a solitary, cold solid (or mixed solid-cystic), hard nodule, a nodule with microcalcifications or irregular edges on ultrasonography; the presence of ipsilateral palpable nodes or vocal cord palsy; and a fine-needle aspiration biopsy (FNAB) suspicious for cancer. Approximately 40% of individuals with a history of therapeutic radiation exposure or a family history of thyroid cancer have a thyroid cancer. The cancer is in the index nodule in 60% and in other nodules in 40% of patients.
Patients with thyroid nodules should be evaluated with TSH measurement and FNAB. If the biopsy suggests a follicular neoplasm, and the TSH level is suppressed or is in the low-normal range, an RAI scan should be performed to rule out a hot nodule.
Nodules with any of the worrisome features mentioned above should be removed with—at minimum—an ipsilateral lobectomy and isthmusectomy. If FNAB confirms a malignancy, the patients should undergo thyroidectomy. Patients with benign nodules on FNAB may be treated with exogenous thyroxine. Nodules that continue to enlarge during follow-up may be re-biopsied or removed via a thyroidectomy.
Malignant thyroid tumors include differentiated lesions (which arise from follicular cells); MTC; undifferentiated or anaplastic cancers; and other rare tumors such as lymphomas, squamous cell carcinomas, sarcomas, teratomas, plasmacytomas, paragangliomas, and metastatic thyroid cancers (from melanoma or from breast, kidney, lung, and other head and neck tumors).
This group includes papillary, follicular variant of papillary, follicular, and Hürthle cell tumors. Hürthle cell carcinoma has been considered a subtype of follicular carcinoma by some investigators and a unique differentiated thyroid cancer of follicular cell origin by others.
The characteristics of these tumors are depicted in Table 26–2. The biologic behavior of the follicular variant of papillary thyroid cancer is similar to that of papillary carcinoma. In follicular and Hürthle cell tumors, the diagnosis of malignancy can only be made by the presence of capsular, blood vessel, or lymphatic invasion or when lymph node or distant metastases are present. Familial non-MTCs, especially if there is a family history of more than two affected relatives, have been shown to be more aggressive than their sporadic counterparts.
| Feature | Papillary | Follicular | Hürthle |
|---|---|---|---|
| Frequency | 80% | 10%-20% | 3%-5% |
| Age group (y) | 20-30 | 40-50 | 50-60 |
| Multicentric | 85% | 10% | 30% |
| Lymph node metastases | 30%-40% | 10% | 25% |
| Distant metastases | 2%-14% | 33% | 15% |
| RAI uptake | 70% | 80% | 10% |
| Prognosis (10-y survival) | 95% | 85% | 65% |
Occult or minimal papillary carcinomas (<1 cm) have an excellent prognosis and are adequately treated by lobectomy. Lobectomy alone is also considered sufficient treatment for low-risk, unifocal, intrathyroidal cancers in the absence of a history of head and neck irradiation or a family history of thyroid cancer or cervical node metastases. Patients with high-risk cancers (determined by AGES, AMES, or TNM classification; Table 26–3, 26–4, and 26–5) or bilateral cancers are best treated by total or near-total thyroidectomy. Considerable debate has existed regarding optimal treatment for low-risk differentiated thyroid cancer >1 cm.
| Variable | Description |
|---|---|
Age | Women older than 50 y Men older than 40 y |
| Grade | Poorly differentiated Fibrous stroma Insular, mucoid, and tall cell variants |
| Extent | Invasive into adjacent tissues or distant metastases |
| Size | Tumor with a maximum diameter of >4 cm |
| Papillary or Follicular | |||
|---|---|---|---|
| Under 45 y | |||
| Stage I | Any T | Any N | M0 |
| Stage II | Any T | Any N | M1 |
| 45 y and older | |||
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T3 T1 T2 T3 | N0 N0 N0 N0 N1a N1a N1a | M0 M0 M0 M0 M0 M0 M0 M0 |
| Stage IVA | T4a T4a T1 T2 T3 T4a | N0 N1a N1b N1b N1b N1b | M0 M0 M0 M0 M0 M0 |
| Stage IVB | T4b | Any N | M0 |
| Stage IVC | Any T | Any N | M1 |
Proponents of total thyroidectomy argued that this procedure is advantageous for several reasons: (1) RAI can be used to diagnose and treat recurrent or metastatic disease, (2) serum thyroglobulin becomes a sensitive indicator of recurrent disease, (3) the procedure eliminates the risk of growth of occult cancer in the contralateral lobe and reduces the risk of recurrence, (4) it decreases the 1% risk of progression to undifferentiated cancer, and (5) it decreases the risk of reoperation in case of central neck recurrence. On the other hand, those favoring thyroid lobectomy noted that (1) total thyroidectomy is associated with a higher complication rate and that 50% of local recurrences can be cured with surgery, (2) <5% of recurrences occur in the thyroid bed, (3) multicentricity within the thyroid is not clinically significant, and (4) the prognosis is excellent in patients with low-risk tumors undergoing lobectomy.
Retrospective data indicate that the recurrence rate for patients with low-risk differentiated thyroid cancer is 10% and that the overall mortality rate is approximately 4% at 10 to 20 years. However, among patients who have recurrences, 33% to 50% die from thyroid cancer. Recent studies involving >50,000 patients with papillary cancers demonstrated that total thyroidectomy is associated with improved recurrence and survival rates even in patients with low-risk tumors. Furthermore, the most important information regarding risk for recurrence is available only postoperatively. Revised guidelines from the American Thyroid Association also recommend near-total or total thyroidectomy for thyroid cancers >1 cm unless there are contraindications to this procedure.
It is not usually possible to distinguish follicular and Hürthle cell carcinomas from corresponding adenomas preoperatively. If there are no obvious signs of cancer at surgery (lymphadenopathy or extrathyroidal invasion), a lobectomy is performed, because more than 80% of these tumors are benign. If final pathology confirms cancer, a completion thyroidectomy is usually recommended, except in those patients with minimally invasive tumors. Total or near-total thyroidectomy may be performed at the initial operation in individuals with large (>4 cm) tumors, particularly if nodules are present bilaterally. Total or near-total thyroidectomy is also recommended if marked atypia is seen on FNA, if it is read as suspicious for papillary carcinoma or the patient has a family history of thyroid cancer or a personal history of head and neck irradiation.
Long-term cohort studies by Mazzaferri and Jhiang and others demonstrate that postoperative RAI therapy reduces recurrence rates and leads to improved survival even in patients with low-risk tumors. Several features place patients at increased risk for local recurrences or metastases. These high-risk features include certain histologic subtypes (such as tall cell, columnar, insular, solid variant, and poorly differentiated thyroid cancer), presence of intrathyroidal vascular invasion, or gross or microscopic multifocal disease. According to recently published guidelines from the American Thyroid Association, RAI is currently recommended for patients with known distant metastases, gross extrathyroidal extension of tumor (regardless of size) or tumors >4 cm even in the absence of other high-risk features. Ablation is also recommended for selected patients with 1 to 4 cm tumors with lymph node metastases or other high-risk features. In contrast, RAI is not recommended for patients with unifocal cancers <1 cm in diameter or patients with multifocal tumors (all <1 cm) without additional high-risk features. Thyroxine therapy is not only important as replacement but is given in higher doses to suppress TSH levels (to 0.1 μU/L in low-risk patients and <0.1 μU/L in high-risk patients), thus decreasing the growth stimulus for thyroid cancer cells. The need for TSH suppression must be balanced with the risks of subclinical thyrotoxicosis, such as exacerbation of angina in patients with ischemic heart disease, increased risk for atrial fibrillation, and osteoporosis in postmenopausal women.
This tumor comprises 7% of thyroid malignancies but accounts for approximately 17% of thyroid cancer–related deaths. It arises from the parafollicular (C cells) of the thyroid, which are derived from the neural crest and secrete calcitonin. MTC may be sporadic (75%) or occur in the setting of MEN 2A, MEN 2B, and familial non-MEN MTC. In the hereditary setting, the tumors are often bilateral and multicentric (90%). Approximately 50% of patients with MTC have nodal metastases in the central or lateral neck at presentation. All patients presenting with MTC should be screened for pheochromocytomas, hyperparathyroidism, and mutations of the RET proto-oncogene.
In terms of surgical therapy, pheochromocytomas should be treated prior to thyroidectomy to avoid precipitating an intraoperative hypertensive crisis. Total thyroidectomy and bilateral central compartment lymphadenectomy is the treatment of choice due to the high incidence of multicentric disease and ineffectiveness of I-131 therapy for this tumor. An ipsilateral prophylactic lateral neck dissection is recommended for patients with positive central neck nodes and for primary tumors larger that 1 cm.
This tumor type constitutes approximately 1% of all thyroid cancers and is the most aggressive variant. The peak incidence is in the seventh decade of life. Lymph node involvement is early and common (84%), as is local invasion into the larynx, vocal cords, recurrent laryngeal nerve, esophagus, and major vessels. Distant metastases are present in about 75% of patients. The role of surgery is usually limited to palliation of obstruction by tumor debulking and tracheostomy. In patients without advanced disease, total or near-total thyroidectomy can be performed for cure in a minority of cases. External beam radiation and chemotherapy are usually recommended.
Most thyroid lymphomas are of the non-Hodgkin B cell type and often develop in patients with long-standing chronic lymphocytic thyroiditis. Chemotherapy and radiotherapy form the mainstay of treatment, with thyroidectomy and node dissection reserved for palliation of airway obstruction or in patients who do not respond to the above therapies. The thyroid gland is a rare site of metastases from tumors of the kidney, lung, breast, and melanoma. In selected patients, thyroidectomy may increase survival.
Clinically inapparent lymph node metastases may be present in 20% to 90% of papillary thyroid cancers, and a smaller proportion of patients with other tumor subtypes. Several retrospective studies have suggested that lymph node metastases do not have a significant effect on survival in papillary thyroid cancers. Others report that lymph node metastases are associated with poorer survival but only in patients with follicular cancers and papillary cancer >45 years of age. In patients with matted nodes or extranodal invasion, the recurrence rates are higher and the prognosis is worse.
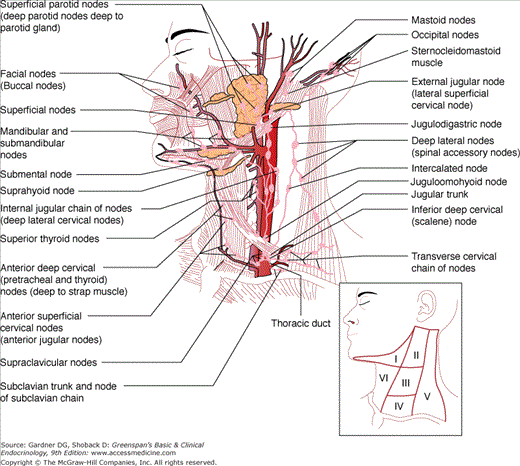
The lymph node regions of the neck are depicted in Figure 26–1. A preoperative neck ultrasound is recommended for patients with malignant findings on FNAB to evaluate the contralateral lobe and lymph node regions. All clinically apparent disease in the central neck should be removed at the time of total thyroidectomy to improve clearance of disease from the central neck. Bilateral central node dissection (level VI) may also improve survival compared to historic controls and decrease the risk of central neck recurrence. Other studies, however, show that central neck dissections are associated with a higher risk of hypoparathyroidism and recurrent nerve injury rates without any reduction in recurrence rates. Given the above, recently published guidelines do not recommend routine prophylactic lymph node dissections but indicate that they may be performed in patients with advanced primary tumors (T3 or T4). Prophylactic central neck clearance should also be considered for Hürthle cell cancers which tend to be less RAI-responsive, provided they can be performed by experienced surgeons without increased rates of hypoparathyroidism and recurrent nerve injury.
Figure 26–1
Lymph node regions of the neck. Level I, submandibular; level II, III, and IV, upper, middle, and lower jugular nodes; level V, posterior triangle nodes; and level VI, central compartment nodes. (Reproduced, with permission, from Roseman BJ, Clark OH. Neck mass. In: Souba W, et al, eds. ACS Surgery—Principles and Practice. 6th ed. WebMD Corporation; 2007.)
Papillary and Hürthle cell cancers can also metastasize to lymph nodes in the lateral and posterior compartments (II, III, IV, and V). In patients with biopsy-proven nodal involvement, either clinically or on preoperative ultrasound examination, an ipsilateral modified radical neck dissection is recommended, particularly if these patients may fail RAI therapy based on lymph node number, size, or aggressive primary tumor histology. This procedure removes all the fibrofatty and lymph node tissue while preserving the internal jugular vein, the spinal accessory nerve, the cervical sensory nerves, and the sternocleidomastoid muscle and may reduce the risk of recurrence and subsequent mortality. Because thyroid cancers rarely metastasize to compartment I, these nodes are not routinely removed during a modified radical neck dissection.
In case of MTC, prophylactic central neck lymph node clearance is recommended because these tumors have a worse prognosis and do not take up RAI. The role of prophylactic lateral neck dissection is controversial. Ipsilateral node removal is recommended for MTCs over 1 cm in diameter (or a palpable tumor) and when the central neck nodes are involved with cancer. If ipsilateral nodes are involved, a contralateral neck dissection will also be needed. Therapeutic modified radical neck dissection is indicated for biopsy-proven metastatic disease in clinically involved nodes or those deemed suspicious by imaging.
Thyroglobulin levels are generally undetectable in patients following total thyroidectomy and RAI ablation. Thyroglobulin levels >2 ng/mL in the absence of thyroglobulin antibodies indicates recurrent or persistent disease. These patients should be imaged with neck ultrasound, CT, or MRI. In general, surgical extirpation is recommended for patients with macronodular disease (>1 cm in diameter), followed by RAI therapy and TSH suppression. Thyroid cancers may metastasize to the lungs, bone, liver, and brain. Microscopic lung metastases in patients with papillary and follicular thyroid cancer are best treated with RAI and macroscopic disease surgically. External beam radiation is useful for unresectable, locally invasive or recurrent disease and to treat bony metastases with minimal to no RAI uptake.
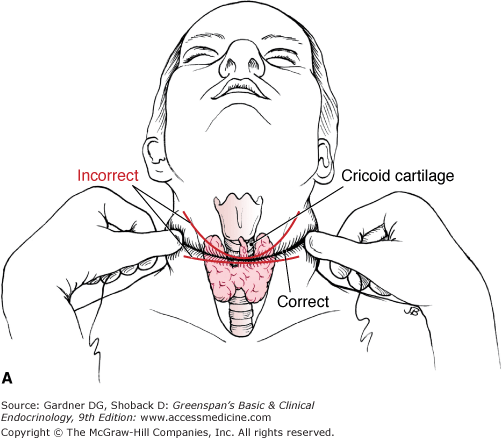
A 4- to 5-cm incision is placed in or parallel to a natural skin crease 1 cm below the cricoid cartilage (Figure 26–2A). The subcutaneous tissue and platysma are divided, subplatysmal flaps are raised (Figure 26–2B), and the strap muscles are separated in the midline from the thyroid cartilage to the suprasternal notch. Initial dissection is begun in the midline by identification of delphian nodes and the pyramidal lobe, followed by division of the fascia just cephalad to the isthmus. The trachea is then cleared just caudal to the isthmus. The thyrothymic ligaments and the inferior thyroid veins are ligated and divided. The side with the dominant or suspicious mass is approached first. In case of a proposed lobectomy or the absence of cancer, the isthmus is divided. Tissues are swept lateral to the thyroid by blunt dissection, and the middle thyroid veins are ligated and divided (Figure 26–2C). The superior pole vessels are then individually ligated and divided low on the thyroid gland to decrease the risk of injury to the external branch of the superior laryngeal nerve (Figure 26–2D). The recurrent laryngeal nerve and the superior parathyroid gland are identified at the level of the cricoid cartilage. Once this is accomplished, the ligament of Berry is divided (Figure 26–2E), and the thyroid is sharply dissected off the trachea. The same procedure is repeated on the other side for a total thyroidectomy.