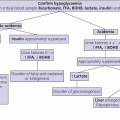
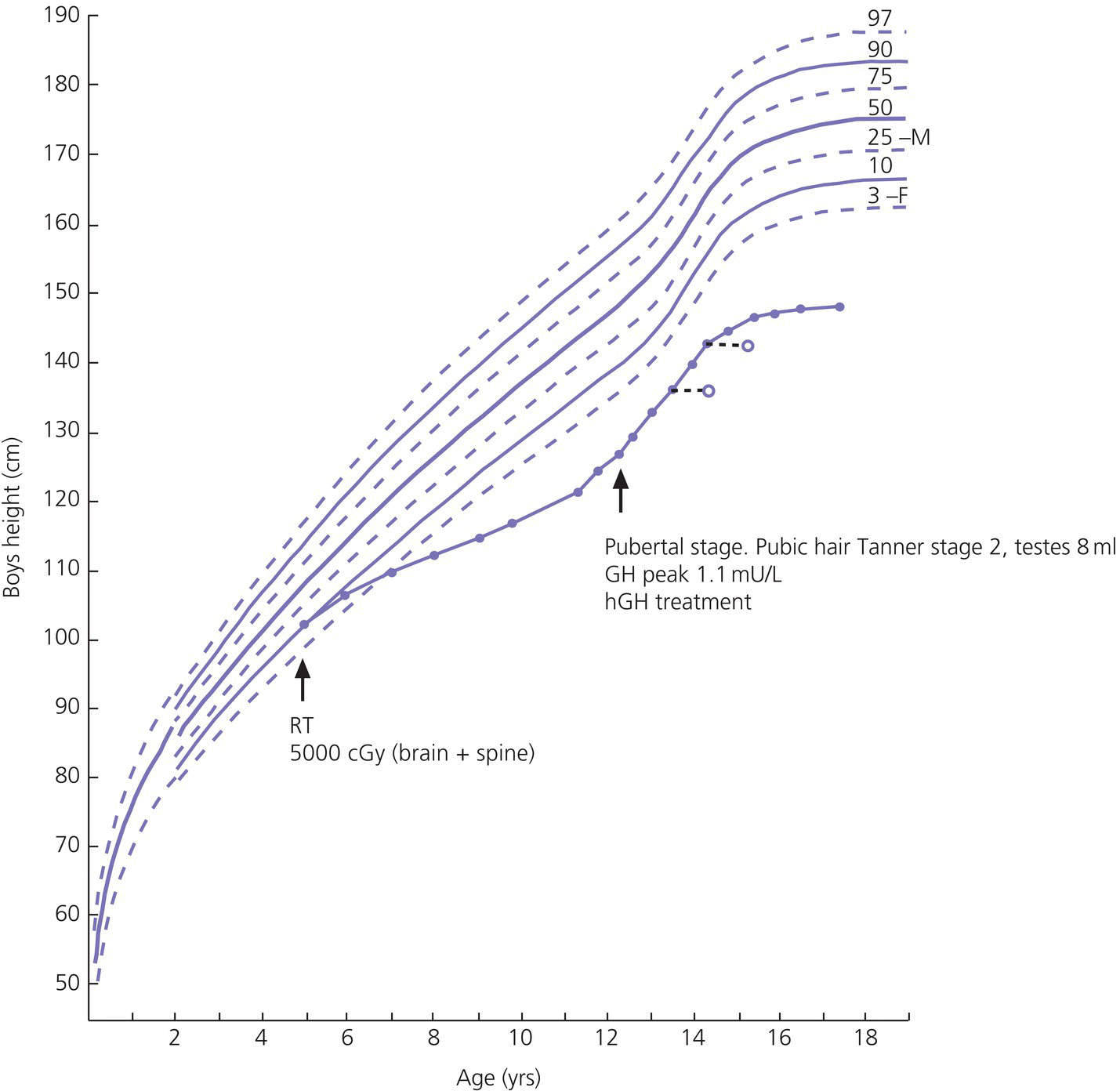
12 There has been a remarkable increase in the survival rates of children with most malignancies during the past 50 years. This improvement has resulted from the use of sophisticated regimens of multi‐agent chemotherapy, frequently combined with radiotherapy and surgery. Treatment of childhood cancer is intensive and associated with effects on a number of organs, of which the endocrine system is particularly vulnerable. The risk of late endocrine effects in survivors is related more to the treatment received than to the nature of the underlying malignancy. Organs related to endocrine function or growth which are susceptible to the effects of cancer treatment are shown in Table 12.1. Table 12.1 Organs related to endocrine function or growth which are susceptible to damage from cancer treatment. Cytotoxic chemotherapy may damage normal developing cells and the damage is dependent on the type and dose of chemotherapeutic agent used. The germinal epithelium in the testis is highly susceptible to alkylating agents, such as cyclophosphamide. Similarly, the potential for normal ovarian function may be impaired with intensive chemotherapy. Many regimens include high‐dose glucocorticoids, which induce iatrogenic Cushing’s syndrome. Radiotherapy, used either alone or in combination with chemotherapy or surgery, is effective in treating a number of childhood cancers. However, the ‘price of cure’ may be high in terms of endocrine function and growth. The potential for damage is related to the dose of radiotherapy delivered, the protocol of delivery, and the site of the primary lesion. Direct damage to the growth plates may also result from spinal or total body irradiation. Damage to the hypothalamo–pituitary axis is relatively common following irradiation of tumours of the brain, face, orbit, and adjacent areas. The thyroid is susceptible to head and neck irradiation. Similarly, the prepubertal testis – often the site of relapse in leukaemia – is highly susceptible to direct radiotherapy. Surgery may represent an additional risk factor for these children. Any neurosurgery in the area of the hypothalamo–pituitary axis may impair signalling between these glands which may present relatively acutely but transiently (e.g. postoperative diabetes insipidus) or in the longer term (e.g. ‘hypothalamic’ obesity). Self‐evidently, surgery which involves removal of substantial proportions of a gland may also place the child at risk of subsequent endocrine deficiency. Figure 12.1 describes all the people who should be involved in a ‘late effects’ clinic. The specific organizational structure will vary among institutions, but oncologists should be encouraged to work closely with paediatricians and paediatric endocrinologists to establish a joint clinic aimed at identification, investigation, and management of late endocrine effects. The principal aim of the oncologist is to treat and monitor the status of the patient’s primary disorder. The endocrinologist can support this process by contributing knowledge and experience of potential and actual endocrine and growth dysfunctions. Monitoring of growth and puberty is an essential function of this clinic. Figure 12.1 Suggested model for a late effects clinic. Late referral to this clinic can lead to considerable delay in diagnosis of treatable endocrine effects. Consequently, all patients who have received radiotherapy in childhood to any organ should be referred one year after completion of treatment. In this way, growth monitoring can identify early growth failure and potential abnormalities of puberty. Patients treated with chemotherapy, which can cause potential endocrine dysfunction, should also be referred. Any oncology patient with concerns about growth, puberty, or changes in body composition, for example, obesity, can benefit from early referral to this clinic. Procedures which may be undertaken either in or associated with the late effects clinic are shown in Table 12.2. Table 12.2 Procedures in or associated with the joint oncology‐endocrine clinic. Cancer treatment may affect the function of a number of specific endocrine organs and tissues related to growth. Frequently, several organ systems are affected in the same patient. The major endocrine organs at risk and growth abnormalities are described individually. Growth can be impaired by: Spinal irradiation, as given for conditions such as medulloblastoma (Figure 12.2) and germinoma, will seriously affect spinal growth, particularly when radiotherapy is given in early childhood. Spinal growth is an essential component of the adolescent growth spurt. Consequently, short stature with disproportion because of shortness of the trunk is frequently seen in these patients. Figure 12.2 Growth following radiotherapy to brain and spine for medulloblastoma. Note the severe height loss because of spinal irradiation, the rather early puberty and the adult height much lower than target, despite GH treatment. RT, radiotherapy. Damage to the hypothalamo–pituitary axis resulting in growth hormone deficiency (GHD) can occur following radiotherapy given in: The prevalence and severity of GHD are related to the total dosage of radiotherapy and the fractionation schedule. The same total dosage given in a larger number of smaller fractions is likely to be less damaging. Radiotherapy regimens for brain tumours and tumours of the face and orbit, which deliver doses >3000 cGy to the hypothalamic–pituitary axis, will almost always result in GH deficiency. This is usually present by two years after treatment. The frequency of overt GHD following prophylactic CNS irradiation and after total body irradiation is variable. However, several studies have reported a prevalence of up to 50%. In addition, the physiological increment in GH secretion at puberty does not occur. A high index of suspicion is important when children at risk of GHD are seen in the late effects clinic. Children should undergo GH stimulation testing at the earliest suspicion of a subnormal height velocity. For an interpretation of the results, see Chapter 3. The pubertal status must always be assessed and considered when interpreting the growth curve. Puberty will stimulate growth and, particularly when precocious, can mask coexistent GH deficiency. Of note, precocious boys who have received alkylating agent may not show the expected increase in testicular volume, so that sequential plasma testosterone levels (preferably at 8.a.m.) are required. The same principles apply to the treatment of irradiation‐induced GH deficiency as with other aetiologies. There is no evidence that GH therapy increases the risk of recurrence of the malignancy after remission has been induced. Radiotherapy is a well‐recognized cause of direct damage to gonads and bone. Sex steroid deficiency will be discussed in section ‘Gonadal Damage’. Growth failure in the absence of direct spinal irradiation or GH deficiency has also been described. The mechanism of this apparent effect of chemotherapy is not clear. It is possible that the growth plates in the spine and long bones are susceptible to damage from intensive chemotherapy. The GH axis is most vulnerable to early damage by radiotherapy. Comparatively, other anterior pituitary hormones are resistant, but central hypogonadism, hypoadrenalism, and hypothyroidism (in order of decreasing frequency) may occur throughout life. This phenomenon was first reported in the 1980s and is now a recognized complication, particularly in girls. Children who received cranial irradiation, particularly at a young age, have a significantly earlier onset of puberty with respect to chronological age and bone age. The combination of GH deficiency and early puberty severely reduces growth potential. In this situation, puberty should be suppressed using gonadotrophin‐releasing hormone (GnRH) analogue therapy to allow maximum benefit from GH replacement. Gonadal damage in childhood can occur following chemotherapy or radiotherapy. There are specific differences affecting the testis and ovary, so each will be described separately. The use of chemotherapeutic regimens which include alkylating agents, such as cyclophosphamide, chlorambucil, and the nitrosoureas, in addition to procarbazine, vinblastine, cytosine arabinoside, and possibly cis‐platinum, are likely to cause damage to germinal epithelium, resulting in azoospermia and infertility in adult life. This has been reported particularly in leukaemia and Hodgkin’s disease. Chemotherapeutic regimens are now being modified with the aim of minimizing this complication. Elevation of basal follicle‐stimulating hormone (FSH) is a useful guide to damage of the germinal epithelium. Testosterone production from Leydig cells is generally not affected. The testis is vulnerable to radiation damage, which in turn depends on the total dosage and dose per fraction. Following total body irradiation (dose 1200–1500 cGy) for leukaemia in prepubertal children, Leydig cell function is not affected but azoospermia may occur. After direct testicular irradiation, testosterone production is also severely reduced. Consequently, it is important to assess testicular size and function in order to define the need for testosterone replacement. Long‐term androgen therapy may be indicated to ensure satisfactory pubertal development and normal testosterone levels in adult life. The ovary is apparently more resistant to damage than the testis. Normal fertility and endocrine function can be expected in adult survivors of childhood leukaemia treatment. Intensive chemotherapeutic regimens for conditions such as Hodgkin’s disease may confer more risk of ovarian dysfunction. High plasma FSH and low AMH (anti‐Müllerian hormone) indicate ovarian damage. The ovary is susceptible to damage from radiotherapy. Direct radiotherapy, or indirect radiotherapy as in total body irradiation, can cause ovarian failure with impairment of ovulation and possible subnormal sex steroid secretion. Vulnerable patients need to be carefully assessed by the endocrinologist and normal pubertal development ensured. After radiotherapy that includes the thyroid area, three main types of thyroid abnormalities may occur: hypothyroidism, benign thyroid nodules, and thyroid cancer. After treatment for Hodgkin’s disease with 3000–5000 cGy to the neck, patients should have their plasma thyroid‐stimulating hormone (TSH) regularly measured and T4 replacement should be started as soon as TSH rises to above normal. Total body irradiation and cranial irradiation are also associated with hypothyroidism, which is often partly central and partly primary, and should therefore be evaluated by measuring both TSH and fT4. Elevation of TSH for long periods of time may confer greater risk of malignancy in the irradiated gland. Thyroid nodules may develop after a long interval and are more likely to be malignant in patients with a history of low dose external radiation. Patients should be reminded to have their neck carefully examined clinically once a year throughout life and any palpable nodule should be promptly evaluated by ultrasound. Although rare, adrenocorticotrophic hormone (ACTH) deficiency may be a late complication of high‐dose (>3000 cGy) cranial irradiation and should be considered if other anterior pituitary hormones are deficient. Survivors of childhood leukaemia and brain tumours often become obese. While surgery and radiotherapy in the hypothalamo–pituitary region may predispose to this condition, other factors likely also play a role. In the longer term, obesity and decreased physical activity are risk factors for the development of the ‘metabolic syndrome’ in adult life. Counselling about this late effect and lifestyle modification to prevent or minimize it is important. Impaired bone mineralization may also occur during childhood and can be demonstrated on dual energy X‐ray absorptiometry (DEXA) scanning. Chronic illness, reduced exercise, glucocorticoid therapy, direct effects of other chemotherapeutic agents on osteoblasts, GH deficiency, impaired sex steroid secretion at puberty, and suboptimal vitamin D intake are all potential factors contributing to reduced bone mineralization. The prompt and accurate diagnosis and treatment of endocrine deficiencies will help to normalize bone mineralization, hence diminishing the risk of osteoporosis in adult life. Many long‐term survivors of childhood cancer who have received treatment known to place them at significant risk of endocrine late effects will remain under follow‐up review in a specialized department. Transfer in late adolescence to a health care facility with expertise in late effects is critical, even for those few patients in whom none has been identified in the paediatric age (see section ‘Transition’). Any patient who is receiving hormone replacement or is known to be at high risk of developing endocrine dysfunction, such that they require regular endocrine testing, should be seen in a clinical service that allows transition of care from a paediatrician to an adult physician with endocrine expertise. In addition, those demonstrating evidence of osteopenia or osteoporosis will require referral to an adult ‘osteoporosis service’. It is also helpful for patients at risk of infertility to be seen for a consultation by adult specialists with expertise in infertility treatment, so that they are counselled about the options available to them later in their adult lives.
Endocrine Effects of Cancer Treatment
Pathophysiology
Hypothalamic–pituitary axis
Growth hormone
Adrenocorticotrophic hormone
Luteinizing hormone/follicle‐stimulating hormone
Thyroid‐stimulating hormone
Antidiuretic hormone (vasopressin)
Thyroid
Testis
Ovary
Spine
Long bones
Growth plates
Breast tissue
Fat mass
Chemotherapy
Radiotherapy
Surgery
Investigation and management of late endocrine effects
The combined oncology–endocrine clinic
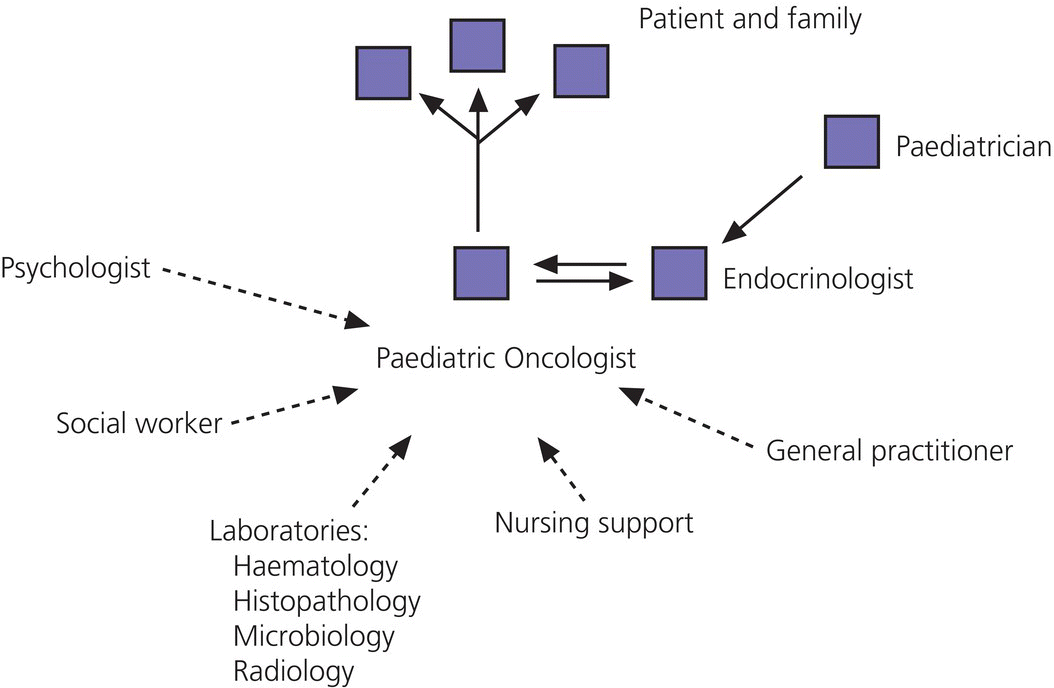
Referral to the late effects clinic
Investigation and monitoring of patients
Clinic attendance – every 4–6 mo
Growth monitoring
Height
Height velocity
Sitting height
Weight
Bone age
Body mass index
Pubertal development staging (including testicular volume)
Hormone measurements
Basal levels: FT4, TSH, cortisol, testosterone, oestradiol, LH, FSH, prolactin, IGF‐I
Dynamic tests: GH stimulation tests, low‐dose ACTH stimulation test, GnRH test
Thyroid: careful palpation and ultrasound if a nodule is suspected
Pelvic ultrasound in girls if puberty is precocious or delayed
DEXA scan for bone mineral density and body composition
Specific endocrine and growth abnormalities associated with cancer treatment
Abnormal linear growth
Spinal irradiation
GH deficiency (GHD)
Diagnosis
Treatment
Direct damage to growth plate and long bones
Abnormal pituitary function
Early puberty following cranial irradiation
Gonadal damage
The testis
Chemotherapy
Radiotherapy
The ovary
Chemotherapy
Radiotherapy
Thyroid abnormalities
Adrenal abnormalities
Abnormalities of body composition
When to involve a specialist centre
Future developments
Transition
Controversial points
Potential pitfalls
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree