The hereditary paraganglioma, MEN1, MEN2, and hereditary thyroid cancer syndromes are clinically discernable and genetically distinct. The first 3 syndromes have been well characterized in the past 10 to 15 years. Recognizing these 3 syndromes and using a multidisciplinary team approach creates valuable opportunities for early diagnosis, reduction of morbidity and mortality, and avoidance of surgical misadventures. Hereditary paraganglioma has parent-of-origin effects and gene-environment interactions that indicate its evolution, and the syndrome sheds light on the role of mitochondria and energy metabolism in cancer. This article delineates the clinical presentation and practical management issues and summarizes the history, gene discovery, and molecular insights for each syndrome.
The hereditary paraganglioma, MEN1, MEN2, and hereditary thyroid cancer syndromes overlap to some extent, but all are clinically discernable and genetically distinct. The first 3 syndromes have been well characterized in the past 10 to 15 years following identification of their molecular underpinnings, and genetic testing plays an indispensable role in clinical management in children and adults. The physician who recognizes these syndromes and uses a multidisciplinary team approach can take advantage of valuable opportunities for early and presymptomatic diagnosis, reduction of morbidity and mortality, and avoidance of surgical misadventures. Hereditary paraganglioma is a fascinating syndrome with parent-of-origin effects and gene-environment interactions that indicate a stamp placed on the human genome by natural selection, and the syndrome sheds light on the role of mitochondria and energy metabolism in cancer. MEN1 probably has the widest pleiotropy of any hereditary cancer syndrome. MEN2 is notable for remarkably precise genotype-phenotype correlations at the level of the codon that determine timing of surgical prevention. This article delineates the clinical presentation and practical management issues and summarizes the history, gene discovery, and molecular insights for each syndrome.
The clinical recognition and description of multiple endocrine neoplasia (MEN) and its subtypes began in the 1900s. The importance of managing patients and families in the context of multidisciplinary teams including genetics professionals cannot be overemphasized for MEN type 1, MEN type 2, and hereditary paraganglioma syndromes.
Hereditary paraganglioma syndrome
The 1933 report by Chase of carotid body tumors diagnosed in 2 sisters in their 20s, 1 of whom had bilateral disease, coupled with a report in the same year of paragangliomas in 3 sisters and several publications in the following decades, shed light on the hereditary nature of paragangliomas, at least in some cases. Autosomal dominant inheritance was suggested in 1949 and in subsequently reported pedigrees. A review of 88 familial and 835 nonfamilial carotid body tumor cases in the literature showed a high rate of bilateral disease in familial (31.8%) versus sporadic (4.4% cases), and an equal sex distribution in familial cases, consistent with autosomal dominant inheritance. A medical record review of 222 carotid body tumors at 12 medical centers in the United States suggested the existence of a multiple primary tumor syndrome in familial cases that manifested bilateral disease, other extra-adrenal paragangliomas, and earlier age of onset. Efforts then concentrated on the collection of families for linkage mapping, culminating in the discovery of the first hereditary paraganglioma gene, SDHD , and candidate gene analyses soon followed, leading more recently to predictive clinical genetic testing, a better understanding of genotype-phenotype relationships and gene-environment interactions, and important insights into the role of mitochondrial metabolism in cancer and the mechanism of parent-of-origin effects seen in the PGL1 and PGL2 syndromes.
Nonchromaffin paragangliomas are rare, occurring in about 1:30,000 to 1:100,000 individuals in the general population. Carotid body tumors are associated with conditions of low oxygen tension, such as emphysema, living at high altitude, and cyanotic congenital heart disease, and cluster in low-altitude countries.
Pathology, Anatomy, and Biochemistry
Pheochromocytomas and extra-adrenal paragangliomas are tumors derived from neural crest tissues or organs, termed paraganglia. Classification is based on anatomic considerations; lesions cannot be distinguished using histologic features. The term pheochromocytoma is reserved by the World Health Organization (WHO) for tumors that arise from chromaffin cells of the adrenal medulla, a prototypical sympathetic nervous system paraganglion. Extra-adrenal paragangliomas derive from either the sympathetic nervous system or the parasympathetic nervous system. The term extra-adrenal pheochromocytoma is imprecise.
The sympathetic nervous system includes the organ of Zuckerkandl (located at the origin of the inferior mesenteric artery and involved in fetal and newborn catecholamine metabolism) as well as chromaffin cells clustered in the paravertebral chain, aortic bifurcation, kidney, liver hila, bladder, and mediastinum. The parasympathetic nervous system is distributed from the middle ear and skull base to the pelvic floor, and includes the carotid body, a tiny organ located at the carotid bifurcation, which is the major oxygen sensor. Sympathetic extra-adrenal paragangliomas are often hormonally active. Parasympathetic extra-adrenal paragangliomas are usually located in the head and neck, and only about 1% are hormonally active.
Terminology for head and neck paragangliomas is varied and includes chemodectoma, carotid body, jugular, vagal, tympanic tumor, and glomus tumor. Pheochromocytomas (of the adrenal medulla) often have an adrenergic profile, whereas extra-adrenal paragangliomas (when active) typically have a noradrenergic profile. Pheochromocytomas are more typical of MEN2A, MEN2B, von Hippel-Lindau syndrome (VHL) (particularly type 2), and neurofibromatosis type 1 (NF1) than hereditary paraganglioma syndromes, but overlap exists. For example, head and neck paragangliomas are an occasional feature of MEN2, VHL, and NF1, and the hereditary paraganglioma syndromes can manifest sympathetic extra-adrenal paragangliomas and pheochromocytomas. Hereditary nonchromaffin paragangliomas are slow-growing, highly vascularized tumors, most of which are benign. The WHO defines malignancy for pheochromocytomas and extra-adrenal paragangliomas not by local invasion but as metastatic spread to distal sites (which are typically devoid of paraganglionic tissue; generally bone, liver, and lung) or clearly identifiable lymph nodes. Adequately resected MEN2 pheochromocytomas do not typically recur, whereas SDHB -related tumors have about a 50% rate of metastasis.
Inheritance and Genetics
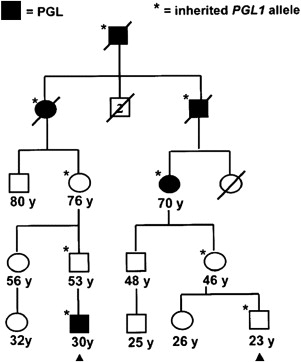
Paraganglioma families typically have an autosomal dominant pattern with age-dependent penetrance. In certain linkage groups, individuals manifesting disease inherit the trait solely through the paternal lineage, suggestive of maternal imprinting. The PGL1 ( SDHD ) and PGL2 loci both show parent-of-origin effects consistent with maternal imprinting. Parent-of-origin effects are shown in Fig. 1 , and have important implications for screening. A single case of an affected offspring following maternal transmission of an SDHD mutation has been reported. The PGL3 ( SDHC ) and PGL4 ( SDHB ) loci follow classic autosomal dominant inheritance for tumor predisposition.
Early linkage studies mapped the PGL1 gene (later shown to be SDHD ) to 11q23-qter, PGL2 ( SDHAF2 / SDH5 ) to 11q13, and PGL3 ( SDHC ) to 1q21-q23. PGL4 ( SDHB ) maps to 1p36.1-p35. Baysal and colleagues identified the first paraganglioma gene in PGL1 kindreds as SDHD , which encodes the D subunit of heterotetrameric succinate dehydrogenase (SDH; mitochondrial complex II) enzyme complex, a component of the respiratory chain and the Krebs cycle. Following this discovery, the other SDH gene subunits became prime candidates for the other genetic loci. Mutations in SDHC occur in PGL3 families and mutations in SDHB in PGL4 families. However, the A subunit of SDH maps to chromosome 5p15, not to the remaining PGL2 locus at 11q13. SDHA mutations are one cause of Leigh syndrome, infantile subacute necrotizing encephalopathy. SDHAF2 / SDH5 assignment to the PGL2 locus was accomplished in yeast models and maps to 11q13, the PGL2 locus.
Clinical Presentation and Diagnosis
Paragangliomas of the head and neck typically present as slowly growing masses that can be asymptomatic for years or even decades. On careful questioning or evaluation, what may seem to be an isolated case can lead to unmasking of a family history of neck mass or other related growth. About 10% of patients present with cranial nerve palsy, most commonly involving the vagal nerve. Left untreated, about 75% of patients develop cranial nerve palsies, providing the potential for presymptomatic diagnosis, intervention, and prevention of serious morbidity. Symptoms include tinnitus, hearing loss, hoarseness, vocal cord paralysis, cough, aspiration, dysphagia, pharyngeal fullness, facial paralysis, problems moving the tongue, or pain. Vagal paragangliomas are located behind the ramus of the mandible and can project into the oropharynx. Magnetic resonance imaging (MRI) is diagnostic based on tumor vascularity and location, characterizes lesions for surgical planning, and assesses multifocality. Incisional biopsy of the highly vascular head and neck paragangliomas that are intimate with neurovascular structures is dangerous, usually unnecessary for diagnosis, and should be avoided; however, when a radiologic diagnosis is unclear, ultrasound-guided fine-needle aspiration can be used.
Pheochromocytomas and functional extra-adrenal paragangliomas may present with the classic triad of episodic headache, diaphoresis, and tachycardia, or with other symptoms. More typically, the classic triad is not seen. Persistent hypertension and normotension are also consistent with the diagnosis of a functional chromaffin cell tumor. Diagnosis of pheochromocytoma can be performed through biochemical testing and localization by imaging. Biochemical testing entails measurement of either 24-hour urine catecholamines and metanephrines or fractionated plasma-free metanephrines; the rationale for choosing one rather than the other has been discussed.
Genotype-Phenotype Correlations, Penetrance, and Expressivity
SDHD and SDHB mutations are more often associated with a positive family history than SDHC mutations. SDHC mutation carriers have a lower risk of pheochromocytoma, multifocality, and malignant transformation. The median age of onset for SDHC is slightly higher than for SDHD and SDHB and the earliest tumor reported in SDHC is at 13 years of age. Age at diagnosis of the first tumor often occurs by age 10 years for SDHB (earliest reported age, 7 years) and penetrance is 25% by age 20 years. Age less than 10 years for a first tumor is rarer, but not unknown for SDHD (earliest reported age, 5 years) and penetrance is 15% by age 20 years. The predominant phenotype for SDHB is extra-adrenal paraganglioma, and renal cell carcinoma is an important sign of SDHB . SDHB is associated with a 41% prevalence of malignant tumors, which may be related to a trend toward malignancy in extra-adrenal paragangliomas. The more severe phenotype of SDHB may be related to its role as part of the catalytic complex. Carriers with nonsense/splicing mutations developed symptoms 8.5 years earlier than missense mutation carriers, and a trend was seen toward pheochromocytoma development with nonsense mutations. Altitude correlates with tumor type. Mutations in the 5′ portion of the SDHD gene are correlated with pheochromocytoma, whereas 3′ mutations are associated with head and neck paragangliomas.
Several reports indicate that renal cell carcinoma is a component tumor for SDHB and renal oncocytoma has also been reported. Thyroid cancer, in particular the papillary type, might be part of the SDH gene spectrum, but this is not well established. In a large fraction of cases, the Carney-Stratakis syndrome (dyad) of paragangliomas and gastrointestinal stromal tumors (GIST) can be attributed to germline SDHD , SDHB , and SDHC mutations. SDHD and SDHB germline mutations or variants have been found in patients with a Cowden syndrome–like presentation negative for PTEN mutations. In these patients, the tumor spectrum seems to include breast, papillary thyroid, and renal cancers.
Diagnostic Criteria for Molecular Genetic Testing
There is an emerging consensus of opinion that all patients with a single head and neck paraganglioma, extra-adrenal paraganglioma, or nonsyndromic pheochromocytoma should undergo molecular genetic testing. Although classic red flags for hereditary cancer still apply, the difficulty arises in using these features to accurately distinguish truly sporadic (ie, non–germline mutated) cases of head and neck paragangliomas from single tumors that have a hereditary basis. Functional paragangliomas that seem to be sporadic have a ~12% rate of mutation detection rate when testing VHL , RET , SDHD , and SDHB , and nonsyndromic pheochromocytoma has a 24% mutation detection rate for RET , VHL , SDHD , and SDHB , with SDHD and SDHB as important contributors.
Regarding single head and neck paragangliomas, individuals with any hereditary features should undergo a genetic workup; that would include any individual with bilateral or multifocal head and neck paragangliomas, a single extra-adrenal paraganglioma in combination with a pheochromocytoma, or early-onset disease. Male gender can be considered an indication for testing. Sporadic head and neck paragangliomas occur more commonly in women, and a large study of head and neck paragangliomas found a 53% mutation detection rate for SDHD , SDHB , and SDHC testing in men. Malignant paraganglioma is also suggestive of a genetic cause, and the prevalence of an underlying SDHB mutation is not affect by a syndromic presentation or positive family history.
Some studies have demonstrated a high genetic contribution to seemingly sporadic extra-adrenal paragangliomas, indicating that there would be many missed cases. Parent-of-origin effects and reduced penetrance contribute to a low specificity of family history, which may be compounded by lack of family history information, unrecognized disease in relatives, and lifelong hereditary risk that extends into the diagnosis age range of sporadic tumors. A decade ago, common wisdom held that only about 10% of head and neck paragangliomas (outside the Netherlands, where the rate is much higher because of founder mutations) had a genetic cause, but soon after the identification of the SDH genes a significantly higher hereditary proportion was better appreciated. SDHD , SDHB , and SDHC mutations contribute to 9.5% of apparently sporadic, 71.4% of multiple cases, and 87.8% of familial head and neck paragangliomas. A genetic workup should thus be considered for most, if not all, head and neck paragangliomas and all malignant pheochromocytomas and extra-adrenal paragangliomas.
Differential Diagnosis
The VHL, MEN2, and NF1 syndromes each have anecdotal reports of head and neck paragangliomas. Paraganglioma, gastric stromal tumor, and pulmonary chondroma comprise the Carney triad, which has an unknown genetic basis. NF1 should be recognizable as a genodermatosis, and VHL and MEN2 are usually evident based on family history or clinical features. After exclusion of SDH genes, only about 0.5% of patients with head and neck paragangliomas have VHL mutations, whereas RET and NF1 mutations are rarer still, suggesting that VHL and RET testing could be foregone.
Patients with a single nonsyndromic pheochromocytoma or extra-adrenal paraganglioma can be stratified for testing (eg, SDH gene testing for head and neck paraganglioma); SDHB initially for thoracic-retroperitoneal paraganglioma, and VHL / SDHB / RET algorithms for pheochromocytoma. Renal cell carcinoma in combination with an extra-adrenal paraganglioma could suggest VHL, but, in the absence of other VHL features in the proband and family, SDHB testing could first be considered.
Diagnostics
DNA sequence analysis and deletion/duplication analysis are available on a clinical basis for SDHD , SDHB , and SDHC , but not SDHAF2 / SDH5 . The proportion of mutation types has been well summarized ; about 3% to 7% comprise large deletions that require analysis by multiplex ligation-dependent probe amplification (MLPA) or similar methods. Sensitivity of molecular genetic testing is probably about 70% to 90%. A study employing SDHB immunohistochemical (IHC) staining in 220 pheochromocytomas and paragangliomas showed that 6 tumors were SDHB IHC negative among 53 tumors without any detectable germline mutation, and 5 of the 6 cases had syndromic features. In the larger series, 102 tumors had detectable SDH mutations. This suggests a mutation detection rate of about 94% (102/108) for SDH gene testing using DNA sequencing and MLPA methodologies.
Because testing for numerous genes for head and neck paragangliomas is costly ($2700 for SDHD , SDHB , and SDHC in the United States); prioritization of genetic testing should be based on first-step predictors of positive family history, preceding pheochromocytoma, multiple tumors, malignant tumors, age 40 years or younger, and male gender which have a predicted sensitivity of 92% and predicted specificity of 94%. An approach to case stratification using IHC staining of SDHB in formalin-fixed paraffin-embedded pheochromocytomas and paragangliomas, analogous to tumor testing of mismatch repair proteins to identify Lynch syndrome, has also been presented.
Prognosis and Management
Treatment of head and neck paragangliomas is mainly surgical, but watchful waiting or radiation therapy may be the best approach for certain tumors such as slowly growing vagal and jugular fossa paragangliomas in older patients in whom surgery may be problematic. Because multicentricity is common, debility from cranial neuropathy is greatly magnified, thus all patients, including those with a single tumor, must undergo preoperative MRI of the head and neck, which also guides surgical planning. It is particularly important to spare at least 1 vagal nerve and its laryngeal branches. Although functional head and neck paragangliomas are rare, preoperative biochemical screening for catecholamines and metanephrines is strongly advised to avoid the catastrophic cardiovascular collapse that can be precipitated by surgery. Biochemical screening also assesses unrecognized or asymptomatic pheochromocytomas and functioning extra-adrenal paragangliomas that occur at significant rates with all of the SDH syndromes.
For patients with pheochromocytomas and extra-adrenal paragangliomas, preoperative achievement of β-adrenergic blockade and maintenance of adequate volume status, as well as intraoperative monitoring and surgical approaches, are detailed elsewhere. Surgical treatment of pheochromocytoma is usually curative, but follow-up testing is needed. Pheochromocytomas and extra-adrenal paragangliomas have an overall 5% to 15% rate of malignancy, but the risk of extra-adrenal paragangliomas and malignancy is higher in SDHB carriers. SDHB mutation has been found to be an independent predictor of mortality for malignant pheochromocytoma or extra-adrenal paraganglioma (relative risk 2.7). It has been suggested that SDHB testing be performed in all patients with malignant pheochromocytomas, and that carriers be more aggressively managed using a combination of surgery, metabolic radiotherapy (eg, 131 I-labeled m -iodobenzylguanidine or radioactive somatostatin analogues) with consideration of chemotherapy. Further details on diagnosis, localization, and treatment have been summarized elsewhere.
There has been no consensus conference outlining screening recommendations for the hereditary paraganglioma syndromes. One regimen is for head and neck MRI every 2 years beginning at age 16 years, along with urinary screening for pheochromocytoma every 2 years. Another recommends annual MRI of the neck, thorax, and abdomen/pelvis with [ 18F ]fluorodopa or [ 18F ]fluorodopamine positron emission tomography ( 18 F DOPA PET) as a possible alternative, coupled with screening for catecholamines and metanephrines. Scans can also be performed with 111 indium pentetreotide scintigraphy, a somatostatin analogue that binds to somatostatin type 2 receptors usually present in paragangliomas, coupled to a radioisotope. This method can detect small (5–10 mm) lesions with high accuracy. 18 F DOPA PET has higher sensitivity than MRI for lesions of less than 1 cm, and whole-body screening is a possible advantage, especially for SDHB carriers. The 3.4% prevalence of renal malignancy in SDHB opens the possibility for screening, particularly in families with this presentation. Age of onset may extend to less than 10 years, especially for SDHB . Genetic testing has been recommended to begin as early as age 5 to 10 years in SDH families, with annual screening in mutation carriers. The later age of onset seen across case series of SDHC carriers suggests that genetic testing and screening could be delayed.
Genetic Function and Genomic Imprinting
Mitochondrial complex II, the only complex in the respiratory chain completely encoded by nuclear genes, is a tetrameric protein encoded by 3 paraganglioma genes, SDHD , SDHB , and SDHC , and a fourth gene, SDHA , which is a cause of Leigh syndrome. The recently discovered paraganglioma gene, SDHAF2 / SDH5 , is required for flavination of the complex, as shown in yeast. Downregulation of SDH results in induction of the hypoxic pathway, and SDH has been proposed as an oxygen sensor, perhaps through the production of reactive oxygen species. PGL1 ( SDHD ) and, apparently, PGL2 ( SDHAF2 / SDH5 ) kindreds both show parent-of-origin effects, whereby carrier offspring of carrier fathers, but not carrier mothers, manifest disease with age-dependent penetrance liabilities. This observation was noted before linkage or cloning of the underlying genes and has held true with more direct evidence from mutational analysis, save for 1 reported exception.
The molecular basis of this parent-of-origin effect remains uncertain but models have been proposed. One possibility is that the SDHD gene is not imprinted, but that selective somatic loss of the maternal wild-type SDHD allele is driven by loss of gene(s) at 11p15 that undergo imprinting. This would constitute a single hit, leaving only paternally imprinted 11p15 gene(s) that are in phase with paternally inherited SDHD mutations, thereby mimicking SDHD imprinting. If PGL2 is more convincingly shown to have a parent-of-origin effect, the same mechanism could be used to explain this effect given its location on chromosome 11.
The role of these genes in neoplasia is also being studied. SDHD was the first tumor suppressor gene identified that encodes a protein involved in mitochondrial metabolism. A second example of a tumor suppressor gene with a role in mitochondrial metabolism is the fumarate hydratase ( FH ) gene. Cancer cells may gain a selective advantage by diverting glucose away from energy production and toward the production of macromolecules used to generate fatty acids, nonessential amino acids, and nucleotides (consistent with the Warburg effect first observed in 1924).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree