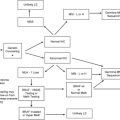
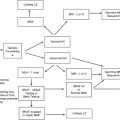
Malignancies of the upper gastrointestinal tract form a heterogeneous group of cancers characterized by unique epidemiology and biology. Despite these differences, survival for advanced disease remains poor across the panel of diseases, from cancers of the esophagus, stomach, pancreas, and, until recently, even gastrointestinal stromal tumors. Genetic predisposition syndromes associated with these diseases comprise an emerging subset of these diseases that may provide valuable information on cause and etiology. They may provide insight into molecular drivers for the disease, or disease subtypes, and also insights into novel gene/environment interactions. This review summarizes the current understanding of genetic predisposition syndromes of cancers of the upper gastrointestinal tract.
Upper gastrointestinal malignancies encompass a heterogenous group of cancers that carry the worst prognosis of solid tumor malignancies of the entire gastrointestinal tract. Cancers of the esophagus, stomach, pancreas, and gastrointestinal stroma are commonly diagnosed in an advanced stage, when treatment goals are for disease control rather than cure. Although they have varying epidemiology and biology, there are several genetic predisposition syndromes that carry an increased risk for these malignancies, many of which are overlapping.
This review highlights the known genetic predisposition syndromes associated with upper gastrointestinal malignancies. Genetic risk may be grouped by family history, high-penetrance alleles, and low-penetrance polymorphisms. Low-penetrance polymorphisms for upper gastrointestinal malignancies are often associated with gene/environment interactions. This article highlights the known genetic risks for developing the each of these individual diseases in the context of these broad categories to provide a clinically relevant, comprehensive review of upper gastrointestinal genetic predisposition syndromes.
Cancer of the esophagus
During the past 50 years, the epidemiology of esophageal cancer has changed considerably. Historically, squamous cell carcinoma (SCC) of the esophagus accounted for more than 90% of the newly diagnosed cases, with common risk factors including heavy tobacco and alcohol use. Since the 1970s, the incidence of adenocarcinoma of the distal esophagus has increased by 4% to 10%/y, and has recently eclipsed the SCC of the esophagus. The risk factors for esophageal adenocarcinoma are obesity, gastroesophageal reflux disease, and the associated pathologic changes characteristic of Barrett esophagus. Annually, approximately 14,000 new cases of esophageal adenocarcinoma and SCC are diagnosed in the United States, with a high ratio of fatalities to cases (0.89). This article reviews the limited knowledge of genetic predisposition syndromes associated with esophageal cancer, highlighting tylosis, BRCA2 kindreds, familial Barrett esophagus, and low-penetrance genetic polymorphisms linked with the development of esophageal carcinoma.
Tylosis: Association with Esophageal SCC
Tylosis is a rare autosomal dominant skin disorder characterized by hyperkeratosis of the palms and soles. This condition has also been associated with the development of esophageal SCC, initially observed in large pedigrees from the United Kingdom and from the United States that demonstrate an autosomal dominant inheritance pattern with complete penetrance of the skin changes by the age of 20 years. The estimated lifetime risk of developing esophageal cancer ranges from 40% (United States pedigree) to 92% (United Kingdom pedigree) by the age of 70 years.
Linkage analysis identifies a 42.5 kb region on chromosome 17q25 that is strongly linked with the disease. Recently, downregulation of cytoglobin gene expression has been associated with the development of tylosis. The proposed mechanism of a germline mutation causing disease is novel: impaired cytoglobin gene transcription and the altered expression of an associated, but as yet unidentified, target gene may be caused by the production of aberrant RNA that is capable of reduced transcription but also by interaction with the cytoglobin gene product.
BRCA2 : A Linkage with Esophageal SCC?
BRCA2 was identified in families with breast cancer who did not have a mutation in BRCA1 and had a high incidence of male breast cancer. BRCA2 , located on chromosome 13q12 to 13, encodes a large 3418–amino-acid protein and is believed to be important in facilitating repair of double-stranded breaks by binding directly to the Rad51 protein and recruiting this recombinase protein to sites of DNA damage. This protein has been associated with familial kindreds of SCC of the esophagus in high-risk areas in the Shanxi Province in China and among the Turkmen population of Iran. In the study from the Shanxi Province, investigators identified 5 germline mutations (4 missense and 1 deletion) among 44 patients with esophageal cancer all with a family history, whereas no germline BRCA2 mutations were identified among the cases without a family history ( P = .078). In the Turkmen study from Iran, a single nonsense variation (K3326X) was identified in 9 patients with squamous cell cancer of the esophagus versus 2/254 controls (odds ratio [OR] 6.0, 95% confidence interval [CI] 1.3–28), thus further implicating this gene in the development of some familial kindreds of esophageal cancer. However, neither the Chinese missense mutations nor the K3266X alteration are clearly associated with loss of BRCA2 function, raising the possibility that these associations may not be causative. In support of this, in these esophageal squamous cell cancer kindreds with BRCA2 variation, no cases of breast or ovarian cancer were identified.
Familial Barrett Esophagus: Association with Esophageal Adenocarcinoma
Barrett esophagus describes the replacement of the normal squamous lining of the esophagus with metaplastic columnar epithelium as a result of chronic gastric acid reflux. Barrett esophagus associated with high-grade dysplasia is considered a premalignant condition with the annual risk for transformation estimated at 1% to 2%. Although traditionally believed to be an acquired condition, there is increasing evidence that a subset of patients with Barrett esophagus may have a genetic predisposition to developing the disease. The risk of developing adenocarcinoma in patients with familial Barrett esophagus seems to be variable, but may be as much as twice that of malignant transformation from sporadic Barrett esophagus.
The familial clustering of Barrett esophagus may be multifactorial, including from shared genes and environmental influences. Twin studies can minimize the confounding of shared environments, and have been examined to identify concordance of Barrett esophagus among monozygotic and dizygotic twins. In 3 separate studies, although the concordance for Barrett esophagus was significantly higher for monozygotic twins than dizygotic twins, the concordance ranged from 19% to 42%, suggesting the presence of additional environmental factors that may modify the penetrance of the disease phenotype.
Low-penetrance Polymorphisms Linked to Esophageal Cancer: Gene-Environment Interaction
Genetic polymorphisms linked to esophageal cancer have been examined for both squamous cell and esophageal adenocarcinoma, evaluating polymorphisms in metabolic pathway proteins or in proteins involved in DNA repair, cell cycle, and apoptosis (reviewed by Cheung and Liu ). Although susceptibility studies across various populations have rarely been consistent, thereby dampening enthusiasm for identification of a true linkage with disease, several studies demonstrate gene-environmental interactions that are highly suggestive of modulation of risk of developing esophageal cancer. For example, 1 polymorphism associated with squamous cell esophageal cancer risk involves the aldehyde dehydrogenase gene, ALDH2 , which is involved in the metabolism of alcohol. Heterozygous individuals, ALDH 1 / 2 , particularly those who have high alcohol consumption, are at a significant risk of developing squamous cell cancer of the esophagus, with an estimated OR of 3.2. Heterozygous individuals who do not drink alcohol, or homozygous ( 2 / 2 ) individuals (typically associated with alcohol intolerance) are at reduced risk of SCC. As another example, homozygous carriers of a DNA repair protein, O(6)-methylguanine-DNA methyltransferase (MGMT) polymorphism (rs12268840) who suffer from acid reflux are at significantly higher risk for developing esophageal adenocarcinoma, with an OR of 15.5. These studies suggest that evaluation of at least some low-penetrance polymorphisms must be taken in the appropriate clinical and environmental context for an association to be identified and, potentially, validated.
Cancer of the stomach
Gastric cancer represents an enormous global health burden. Gastric cancer is the second most common cause of cancer-related deaths worldwide, with 700,349 deaths annually, and is the third most common malignancy worldwide, with 974,000 new cases in the year 2000. Although the incidence of gastric cancer in the United States has declined in the past several decades, it remains a significant health problem throughout southeast Asia, eastern Europe, and Central America. Nearly two-thirds of cases occur in eastern Europe, South America, and Asia, with 42% in China alone. In the United States, an estimated 21,500 new cases (14th most common) of gastric cancer were diagnosed in 2008 with 10,880 deaths (13th most common).
Gastric cancers are often grouped together, although there are considerable clinical and pathologic differences in disease, distinguished by disease location, histology, and molecular characteristics. Virtually all stomach cancers are adenocarcinomas that can be pathologically distinguished according to the Lauren classification as intestinal or diffuse subtypes. Intestinal gastric cancers are generally well differentiated with a glandular appearance, and tend to expand through the stomach wall, whereas diffuse gastric cancers are more commonly poorly differentiated and spread as single discohesive cells that infiltrate throughout the stomach wall. Intestinal gastric cancers are more prevalent in high-incidence areas and are responsible for much of the observed global ethnic variation. In contrast, the incidence of diffuse gastric cancer is approximately the same, independent of geography or race. It is more infrequent than intestinal gastric cancer, occurring at an incidence of 0.3 to 1.8 per 100,000 people. When reviewing the genetics of gastric cancer, it is important to place this information in context with disease biology. As understanding of the biology of this heterogeneous disease improves, the ability to screen for early stage disease and treat more advanced disease should also improve.
Familial Gastric Cancer
A family history of gastric cancer confers an OR of 2.1 to 6 for developing gastric cancer. The risk of developing gastric cancer increases with increasing numbers of family members with the disease, up to an OR of 8.5 when 2 or more siblings are affected, and, in a study of twin siblings, the sibling of a male monozygotic twin who had stomach cancer has an OR of 9.9 for developing gastric cancer, compared with an individual without a twin without gastric cancer. The cause for the high risk of gastric cancer conferred by a familial history is largely unknown. Approximately 15% of all new gastric cancer diagnoses are heritable, whereas the remaining 85% are sporadic.
Hereditary Diffuse Gastric Cancer
Hereditary diffuse gastric cancer (HDGC) is a genetic predisposition syndrome that was defined in 1999 by the International Gastric Cancer Linkage Consortium (IGCLC) as any family that fulfills 1 of the following criteria: (1) 2 or more documented cases of diffuse gastric cancer in first- or second-degree relatives, with at least 1 diagnosed before the age of 50 years; or (2) 3 or more cases of diffuse gastric cancer in first- or second-degree relatives, independent of age. HDGC (Online Mendelian Inheritance in Man [OMIM] # gastric cancer 137215) is an autosomal dominant syndrome characterized by a 60% to 80% penetrance for the development of diffuse-type gastric cancer. Families with HDGC also have a higher prevalence of developing lobular breast cancer and signet ring cell colon cancer. Thus, modified HDGC criteria now also include a family history of 1 of these cancers in addition to early-onset diffuse gastric cancer as sufficient criteria for the syndrome.
HDGC is caused by functional mutations or deletions in the E-cadherin ( CDH1 ) gene on chromosome 16q22. E-cadherin is a calcium-dependent cell-to-cell adhesion molecule on epithelial cells that maintains intercellular adhesion and epithelial cell architecture. Loss of E-cadherin expression has been demonstrated in many sporadic human cancers and is associated with malignancy, invasion, and metastasis. CDH1 is believed to have both tumor suppressor and invasion suppressor functions. A CDH1 mutation was first identified in several Maori kindreds with familial gastric cancer in 1998. To date, more than 70 families with CDH1 mutations have been identified, each with a predominance of early-onset diffuse gastric adenocarcinoma. Many different mutations, rather than a few recurring alterations, have been identified. More recently, CDH1 allelic expression imbalance was seen in 70% of patients with diffuse gastric cancer meeting HDGC criteria who were mutation or deletion negative, suggesting that additional mechanisms of allelic loss of CDH1 are likely present in those familial kindreds without CDH1 mutation or deletion. E-cadherin loss is observed by immunohistochemistry in approximately 50% of patients with sporadic diffuse gastric cancer, suggesting that this pathway may present a possible molecular target to exploit.
There are preliminary clues to a potential racial variance with regard to the occurrence of CDH1 mutations. CDH1 is a highly polymorphic gene with significant variance throughout each of its 16 exons and coding regions, and no regions considered as mutational hot spots. Germline mutations in CDH1 occur in as many as 20% of patients with sporadic diffuse gastric cancer who developed the disease before the age of 50 years. However, in high-incidence areas (ie, Asia/Central America), germline CDH1 mutations in sporadic diffuse gastric cancer are found less often, at about 1% to 2%. Recently, a large registry-based cohort study reported that the incidence of germline CDH1 mutations occurred in 30% of families with a family history of gastric cancer, and in 20% of isolated early-onset diffuse gastric cancer cases. However, this study included only 3 African Americans, and 4 Asian/Pacific Islander families, out of a total of 31 tested, again highlighting the lack of racial/minority representation in this type of registry research. This study was not powered to determine whether the incidence of CDH1 mutations in familial and early-onset gastric cancer differed based on race or ethnicity. The current recommendations for therapy for patients harboring a CDH1 mutation is prophylactic gastrectomy, which is felt to greatly reduce the risk of developing gastric cancer.
Other Germline Genetic Causes of Heritable Gastric Cancer
HDGC represents approximately 30% of all familial gastric cancers. The genetic cause of the remainder is yet to be identified. There has been 1 reported gastric cancer familial kindred having a germline mutation in MET , the genet that encodes for cMET, a transmembrane receptor tyrosine kinase responsible for cellular proliferation, migration, invasion in embryogenesis, and wound healing. This has prompted significant research in cMET signaling as a potential target for gastric cancer, and has led to multiple (ie, >6) novel cMET inhibitors in clinical evaluation, several of which are specifically for gastric cancer. Other rare genetic causes of familial gastric cancer include hereditary nonpolyposis colon cancer syndrome (HNPCC), FAP, Li-Fraumeni syndrome (p53 mutations), and Peutz-Jeghers syndrome (PJS).
Lynch syndrome (also known as HNPCC) is an autosomal dominant condition caused by germline mutations in one of the mismatch repair genes MLH1 , MSH2 , MSH6 , PMS1 , and PMS2 . Microsatellite instability characterizes all of the tumors that arise in association with Lynch syndrome. Endometrial and colorectal cancers are the most commonly described cancers in this syndrome. Lynch syndrome accounts for about 3% of the more than 1 million cases of colorectal cancer that will occur worldwide this year. Stomach cancers arise in approximately 11% of Lynch syndrome families carrying germline mutations in MLH1 , MSH2 , or MSH6 . Most malignancies are Laurens intestinal histology, and have the same natural history as sporadic intestinal gastric cancer.
Familial adenomatous polyposis (FAP) is a condition associated with significant increased development of foregut as well as hindgut adenomatous polyps. Patients with FAP carry a germline mutation in adenomatous polyposis coli ( APC ), a tumor suppressor gene located on chromosome 5q21. Loss of heterozygosity (ie, a second hit) inactivates the second allele and begins the cascade to polyp formation. Subsequent somatic mutations are required, including KRAS and p53 , that in turn lead to progression from an adenoma to a carcinoma. Upper gastrointestinal polyps commonly occur in the gastric antrum (fundic gland polyps) and duodenum. In the stomach, the development of fundic gland polyps does not seem to increase the rate of antral adenocarcinoma in North American or European patients, but may in Japanese or Korean families that carry a germline APC mutation.
Li-Fraumeni syndrome, carrying a germline p53 mutation, is associated with a variety of malignancies, most notably soft tissue sarcoma, leukemia, brain cancer, adrenal cortical cancer, and breast cancer. Oliveira and colleagues describe one family with a p53 germline mutation (R158G, G → C471) with a cluster of predominant gastric cancers. PJS is an autosomal dominant hereditary disease characterized by hamartomatous polyps of the gastrointestinal tract, described more fully later.
Genetics of Intestinal Gastric Cancer: Low-penetrance Polymorphisms and Helicobacter pylori Infection
The development of intestinal gastric cancer, particularly of the body and fundus, is closely related to infection with Helicobacter pylori , a gram-negative bacillus discovered in 1983 to be the pathogen responsible for gastric ulcers and peptic ulcer disease. In 1994, the World Health Organization and the International Agency for Research on Cancer consensus group classified H pylori as a class I carcinogen. However, although H pylori is a common worldwide pathogen, less than 2% of infected patients develop gastric cancer during their lifetime. H pylori is an endemic pathogen with high prevalence rates in both developing and industrialized countries. The bacterium is present in the stomachs of at least half of the world’s population, is usually acquired in childhood, and, when left untreated, generally persists for the host’s lifetime. As such, exposure to this pathogen is chronic and long standing. The rate of H pylori infection is highest amongst the elderly minority population, with a rate of 73% in non-Hispanic blacks, and 74% in Hispanic individuals. In approximately 10% of cases, the pathogen is associated with diverse clinical outcomes, including nonulcer dyspepsia, peptic ulcer disease, and distal gastric cancer.
Several studies have examined low-penetrance polymorphisms and their association with H pylori associated gastric cancer. Although H pylori is a common worldwide pathogen, less than 5% of infected patients develop gastric cancer during their lifetime. The host’s immune response to this pathogen has been implicated in determining the risk of developing gastric cancer, and, in particular, cytokines interleukin (IL)-1 and IL-8, which mediate the inflammatory response to the bacterial infection ( Table 1 ). The IL-1 gene cluster is on chromosome 2q, and contains 3 related genes within a 430-kb region, IL-1A , IL-1B , and IL-1RN , which encode for IL-1α, IL-1β and their receptor antagonist, IL-1ra. IL-1β is a potent proinflammatory cytokine and powerful inhibitor of gastric acid secretion that plays an important primary role in the initiation and amplification of the inflammatory response to H pylori infection. Polymorphisms in the 3′ regulatory region of IL-1B ( IL-1B C-T -551 and -31) and IL-1RN ( IL-1RN2 ) are associated with an increased risk of hypochlorhydria, decreased acid secretion, and are also associated with a significantly higher risk of gastric cancer following H pylori infection. The estimated OR for developing gastric cancer with the IL-1B-31T allele was 1.6 (95% CI 1.2–2.2) and for the IL-1RN2/2 allele was 2.9 (95% CI 1.9–4.4). These IL-1–related polymorphisms and the association with gastric cancer have now been reported in several other populations.
| Gene | Function of Gene Product | Polymorphism | Gastric CA OR |
|---|---|---|---|
| IL-1β | Induces expression of inflammatory cytokines, inhibits gastric acid production | −31 C-T −511 C-T | 2.5 (1.6–3.8) 2.6 (1.7–3.9) |
| IL-1Rβ | Receptor for IL-1β | Penta-allelic 86-bp tandem repeat (IN 2) | 2.9 (1.9–4.4) |
| TNF-α | Activates inflammation and apoptosis signaling pathways; inhibits acid secretion | −308 A/A | 1.9 (1.2–2.8) |
| IL-10 | Inhibits production of proinflammatory cytokines | −591 ATA/ATA −819 ATA/ATA −1082 ATA/ATA | 3.4 (1.4–8.1) |
| IL-8 | T allele increases IL-8 promoter activity | −251 A/T | 2.1–2.52 |
IL-8 is a member of the CXC chemokine family that functions as a potent chemoattractant for neutrophils and lymphocytes. IL-8 levels are increased tenfold in gastric cancer specimens compared with normal gastric tissues. Activation of IL-8 gene expression is mediated by H pylori activation of nuclear factor-κB, a nuclear transcription factor also implicated in the pathogenesis of gastric cancer and a negative prognostic factor for patients with the disease. The A→T promoter polymorphism at -251 ( IL-8 -251T ) has two- to fivefold higher promoter activity than the IL-8 -251A allele, because of high-affinity binding of the -251A allele to a nuclear protein that acts as a negative regulator of promoter function. In a Chinese cohort study, the IL-8 AT or TT genotype (associated with increased IL-8 expression) was significantly associated with an increased risk of gastric cancer. Importantly, this association was identified specifically in patients with diffuse gastric cancer in the setting of prior or current H pylori infection ( IL-8 -251A/T OR 2.12, 95% CI 1.17–3.86; IL-8 -251T/T OR 2.52, 95% CI 1.3–4.9), whereas those patients without H pylori infection had no increased risk of gastric cancer associated with IL-8 -251 T genotype.
Recently, investigators examined 12 meta-analyses and 1 meta-analysis and pooled analysis that were published from 2005 to 2008, focusing on genes involved in inflammation, detoxification of carcinogens ( GSTM1 , GSTT1 , CYP2E1 ), folate metabolism ( MTHFR ), adhesion ( CDH1 ), and cell cycle regulation ( p53 ). Most of the polymorphisms have a small effect in increasing gastric cancer risk, and are of borderline significance or are not significant ( Table 2 ).
| Gene | Meta-OR | Confidence Limits |
|---|---|---|
| CYP2E1 Pst1/Rsa1 c2c2 | 1.36 | 0.82–2.25 |
| GSTM1 null | 1.24 | 1.00–1.54 |
| GSTTf null | 1.09 1.06 | 0.97–1.21 0.94–1.18 |
| MTHFR 677TT | 1.68 1.59 1.52 | 1.29–2.19 1.28–1.98 1.31–1.77 |
| MTHFR 1298CC | 0.90 0.94 | 0.44–1.57 0.65–1.35 |
| p53 codon 72 Arg/Arg | 0.96 | 0.78–1.24 |
| CDH1 -160A | 0.98 | 0.78–1.24 |
Cancer of the stomach
Gastric cancer represents an enormous global health burden. Gastric cancer is the second most common cause of cancer-related deaths worldwide, with 700,349 deaths annually, and is the third most common malignancy worldwide, with 974,000 new cases in the year 2000. Although the incidence of gastric cancer in the United States has declined in the past several decades, it remains a significant health problem throughout southeast Asia, eastern Europe, and Central America. Nearly two-thirds of cases occur in eastern Europe, South America, and Asia, with 42% in China alone. In the United States, an estimated 21,500 new cases (14th most common) of gastric cancer were diagnosed in 2008 with 10,880 deaths (13th most common).
Gastric cancers are often grouped together, although there are considerable clinical and pathologic differences in disease, distinguished by disease location, histology, and molecular characteristics. Virtually all stomach cancers are adenocarcinomas that can be pathologically distinguished according to the Lauren classification as intestinal or diffuse subtypes. Intestinal gastric cancers are generally well differentiated with a glandular appearance, and tend to expand through the stomach wall, whereas diffuse gastric cancers are more commonly poorly differentiated and spread as single discohesive cells that infiltrate throughout the stomach wall. Intestinal gastric cancers are more prevalent in high-incidence areas and are responsible for much of the observed global ethnic variation. In contrast, the incidence of diffuse gastric cancer is approximately the same, independent of geography or race. It is more infrequent than intestinal gastric cancer, occurring at an incidence of 0.3 to 1.8 per 100,000 people. When reviewing the genetics of gastric cancer, it is important to place this information in context with disease biology. As understanding of the biology of this heterogeneous disease improves, the ability to screen for early stage disease and treat more advanced disease should also improve.
Familial Gastric Cancer
A family history of gastric cancer confers an OR of 2.1 to 6 for developing gastric cancer. The risk of developing gastric cancer increases with increasing numbers of family members with the disease, up to an OR of 8.5 when 2 or more siblings are affected, and, in a study of twin siblings, the sibling of a male monozygotic twin who had stomach cancer has an OR of 9.9 for developing gastric cancer, compared with an individual without a twin without gastric cancer. The cause for the high risk of gastric cancer conferred by a familial history is largely unknown. Approximately 15% of all new gastric cancer diagnoses are heritable, whereas the remaining 85% are sporadic.
Hereditary Diffuse Gastric Cancer
Hereditary diffuse gastric cancer (HDGC) is a genetic predisposition syndrome that was defined in 1999 by the International Gastric Cancer Linkage Consortium (IGCLC) as any family that fulfills 1 of the following criteria: (1) 2 or more documented cases of diffuse gastric cancer in first- or second-degree relatives, with at least 1 diagnosed before the age of 50 years; or (2) 3 or more cases of diffuse gastric cancer in first- or second-degree relatives, independent of age. HDGC (Online Mendelian Inheritance in Man [OMIM] # gastric cancer 137215) is an autosomal dominant syndrome characterized by a 60% to 80% penetrance for the development of diffuse-type gastric cancer. Families with HDGC also have a higher prevalence of developing lobular breast cancer and signet ring cell colon cancer. Thus, modified HDGC criteria now also include a family history of 1 of these cancers in addition to early-onset diffuse gastric cancer as sufficient criteria for the syndrome.
HDGC is caused by functional mutations or deletions in the E-cadherin ( CDH1 ) gene on chromosome 16q22. E-cadherin is a calcium-dependent cell-to-cell adhesion molecule on epithelial cells that maintains intercellular adhesion and epithelial cell architecture. Loss of E-cadherin expression has been demonstrated in many sporadic human cancers and is associated with malignancy, invasion, and metastasis. CDH1 is believed to have both tumor suppressor and invasion suppressor functions. A CDH1 mutation was first identified in several Maori kindreds with familial gastric cancer in 1998. To date, more than 70 families with CDH1 mutations have been identified, each with a predominance of early-onset diffuse gastric adenocarcinoma. Many different mutations, rather than a few recurring alterations, have been identified. More recently, CDH1 allelic expression imbalance was seen in 70% of patients with diffuse gastric cancer meeting HDGC criteria who were mutation or deletion negative, suggesting that additional mechanisms of allelic loss of CDH1 are likely present in those familial kindreds without CDH1 mutation or deletion. E-cadherin loss is observed by immunohistochemistry in approximately 50% of patients with sporadic diffuse gastric cancer, suggesting that this pathway may present a possible molecular target to exploit.
There are preliminary clues to a potential racial variance with regard to the occurrence of CDH1 mutations. CDH1 is a highly polymorphic gene with significant variance throughout each of its 16 exons and coding regions, and no regions considered as mutational hot spots. Germline mutations in CDH1 occur in as many as 20% of patients with sporadic diffuse gastric cancer who developed the disease before the age of 50 years. However, in high-incidence areas (ie, Asia/Central America), germline CDH1 mutations in sporadic diffuse gastric cancer are found less often, at about 1% to 2%. Recently, a large registry-based cohort study reported that the incidence of germline CDH1 mutations occurred in 30% of families with a family history of gastric cancer, and in 20% of isolated early-onset diffuse gastric cancer cases. However, this study included only 3 African Americans, and 4 Asian/Pacific Islander families, out of a total of 31 tested, again highlighting the lack of racial/minority representation in this type of registry research. This study was not powered to determine whether the incidence of CDH1 mutations in familial and early-onset gastric cancer differed based on race or ethnicity. The current recommendations for therapy for patients harboring a CDH1 mutation is prophylactic gastrectomy, which is felt to greatly reduce the risk of developing gastric cancer.
Other Germline Genetic Causes of Heritable Gastric Cancer
HDGC represents approximately 30% of all familial gastric cancers. The genetic cause of the remainder is yet to be identified. There has been 1 reported gastric cancer familial kindred having a germline mutation in MET , the genet that encodes for cMET, a transmembrane receptor tyrosine kinase responsible for cellular proliferation, migration, invasion in embryogenesis, and wound healing. This has prompted significant research in cMET signaling as a potential target for gastric cancer, and has led to multiple (ie, >6) novel cMET inhibitors in clinical evaluation, several of which are specifically for gastric cancer. Other rare genetic causes of familial gastric cancer include hereditary nonpolyposis colon cancer syndrome (HNPCC), FAP, Li-Fraumeni syndrome (p53 mutations), and Peutz-Jeghers syndrome (PJS).
Lynch syndrome (also known as HNPCC) is an autosomal dominant condition caused by germline mutations in one of the mismatch repair genes MLH1 , MSH2 , MSH6 , PMS1 , and PMS2 . Microsatellite instability characterizes all of the tumors that arise in association with Lynch syndrome. Endometrial and colorectal cancers are the most commonly described cancers in this syndrome. Lynch syndrome accounts for about 3% of the more than 1 million cases of colorectal cancer that will occur worldwide this year. Stomach cancers arise in approximately 11% of Lynch syndrome families carrying germline mutations in MLH1 , MSH2 , or MSH6 . Most malignancies are Laurens intestinal histology, and have the same natural history as sporadic intestinal gastric cancer.
Familial adenomatous polyposis (FAP) is a condition associated with significant increased development of foregut as well as hindgut adenomatous polyps. Patients with FAP carry a germline mutation in adenomatous polyposis coli ( APC ), a tumor suppressor gene located on chromosome 5q21. Loss of heterozygosity (ie, a second hit) inactivates the second allele and begins the cascade to polyp formation. Subsequent somatic mutations are required, including KRAS and p53 , that in turn lead to progression from an adenoma to a carcinoma. Upper gastrointestinal polyps commonly occur in the gastric antrum (fundic gland polyps) and duodenum. In the stomach, the development of fundic gland polyps does not seem to increase the rate of antral adenocarcinoma in North American or European patients, but may in Japanese or Korean families that carry a germline APC mutation.
Li-Fraumeni syndrome, carrying a germline p53 mutation, is associated with a variety of malignancies, most notably soft tissue sarcoma, leukemia, brain cancer, adrenal cortical cancer, and breast cancer. Oliveira and colleagues describe one family with a p53 germline mutation (R158G, G → C471) with a cluster of predominant gastric cancers. PJS is an autosomal dominant hereditary disease characterized by hamartomatous polyps of the gastrointestinal tract, described more fully later.
Genetics of Intestinal Gastric Cancer: Low-penetrance Polymorphisms and Helicobacter pylori Infection
The development of intestinal gastric cancer, particularly of the body and fundus, is closely related to infection with Helicobacter pylori , a gram-negative bacillus discovered in 1983 to be the pathogen responsible for gastric ulcers and peptic ulcer disease. In 1994, the World Health Organization and the International Agency for Research on Cancer consensus group classified H pylori as a class I carcinogen. However, although H pylori is a common worldwide pathogen, less than 2% of infected patients develop gastric cancer during their lifetime. H pylori is an endemic pathogen with high prevalence rates in both developing and industrialized countries. The bacterium is present in the stomachs of at least half of the world’s population, is usually acquired in childhood, and, when left untreated, generally persists for the host’s lifetime. As such, exposure to this pathogen is chronic and long standing. The rate of H pylori infection is highest amongst the elderly minority population, with a rate of 73% in non-Hispanic blacks, and 74% in Hispanic individuals. In approximately 10% of cases, the pathogen is associated with diverse clinical outcomes, including nonulcer dyspepsia, peptic ulcer disease, and distal gastric cancer.
Several studies have examined low-penetrance polymorphisms and their association with H pylori associated gastric cancer. Although H pylori is a common worldwide pathogen, less than 5% of infected patients develop gastric cancer during their lifetime. The host’s immune response to this pathogen has been implicated in determining the risk of developing gastric cancer, and, in particular, cytokines interleukin (IL)-1 and IL-8, which mediate the inflammatory response to the bacterial infection ( Table 1 ). The IL-1 gene cluster is on chromosome 2q, and contains 3 related genes within a 430-kb region, IL-1A , IL-1B , and IL-1RN , which encode for IL-1α, IL-1β and their receptor antagonist, IL-1ra. IL-1β is a potent proinflammatory cytokine and powerful inhibitor of gastric acid secretion that plays an important primary role in the initiation and amplification of the inflammatory response to H pylori infection. Polymorphisms in the 3′ regulatory region of IL-1B ( IL-1B C-T -551 and -31) and IL-1RN ( IL-1RN2 ) are associated with an increased risk of hypochlorhydria, decreased acid secretion, and are also associated with a significantly higher risk of gastric cancer following H pylori infection. The estimated OR for developing gastric cancer with the IL-1B-31T allele was 1.6 (95% CI 1.2–2.2) and for the IL-1RN2/2 allele was 2.9 (95% CI 1.9–4.4). These IL-1–related polymorphisms and the association with gastric cancer have now been reported in several other populations.