Figure 37.1 Splinter hemorrhage of nails in patient with Staphylococcus aureus infective endocarditis.
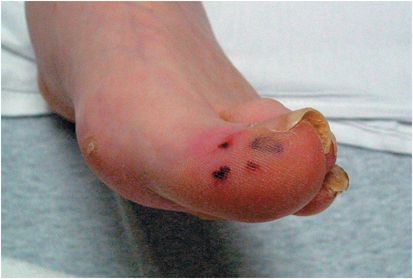
Figure 37.2 Embolic lesions in a patient with Staphylococcus aureus infective endocarditis.
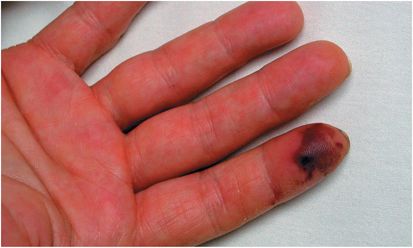
Figure 37.3 Embolic lesions in a patient with Staphylococcus aureus infective endocarditis.
Prosthetic valve endocarditis
The overall incidence of prosthetic valve endocarditis (PVE) is 1% to 4% during the first 12 months following valve surgery. The causative organisms differ in early and late PVE. Infection within 60 days of valve insertion is considered to be early PVE and is usually caused by Staphylococcus epidermidis (25% to 30%) or S. aureus (15% to 20%). In a prospective study of patients with prosthetic valves and S. aureus bacteremia conducted at Duke University Medical Center, 26 of 51 patients (51%) developed PVE. The risk of endocarditis in this study was independent of the type of valve (mechanical vs. bioprosthetic), location (mitral vs. aortic), or onset of bacteremia after prosthetic valve implantation. The remaining cases of PVE are caused by gram-negative aerobic organisms, enterococci, diphtheroids, and streptococci. Fungal endocarditis may develop in patients with prolonged hospitalization with indwelling central venous catheters and long-term antibiotic use. Organisms that cause late PVE closely resemble those of NVE, although staphylococci remain predominant.
Nosocomial endocarditis
IE can occur as a complication of nosocomial bacteremia. Since the mid 1980s, the increased use of intravascular devices and invasive diagnostic procedures, with their consequent complications, has increased the risk of healthcare-associated endocarditis. Colonized intravascular catheters now account for up to two-thirds of healthcare-associated IE. S. aureus, including methicillin-resistant S. aureus (MRSA), S. epidermidis, enterococcus, and Candida species are the predominant pathogens.
The Infectious Disease Society of America has updated the catheter-related bloodstream infection (CRBSI) guidelines, with key recommendations regarding S. aureus BSI (Table 37.1). S. aureus in blood should rarely, if ever, be considered a contaminant and a low threshold should be maintained regarding suspicion of IE in this setting.
| Catheter removal recommended |
|---|
| Long-term catheters should be removed from patients with CRBSI associated with any of the following conditions: endocarditis, severe sepsis, bloodstream infection that continues despite more than 72 hours of appropriate antimicrobial therapy, suppurative thrombophlebitis, or infections due to S. aureus, Pseudomonas aeruginosa, fungi, or mycobacteria |
| S. aureus specific recommendations |
|---|
| In addition to catheter removal, patients should receive 4–6 weeks of antimicrobial therapy, or a minimum of 14 days of therapy if they meet the following criteria: the patient is not diabetic or immunosuppressed; if the infected catheter is removed; if the patient has no prosthetic intravascular device (e.g., pacemaker or recently placed vascular graft); if there is no evidence of endocarditis or suppurative thrombophlebitis on TEE and ultrasound, respectively; if fever and bacteremia resolve within 72 h after initiation of appropriate antimicrobial therapy; and if there is no evidence of metastatic infection on physical examination and sign or symptom-directed diagnostic tests |
Long-term catheters should be removed in the setting of endocarditis. Transesophageal echocardiography (TEE) should be performed (at least 5–7 days after the onset of bacteremia or fungemia) for patients with CRBSI who have a prosthetic heart valve, pacemaker, or implantable defibrillator.
Infective endocarditis in the intravenous drug abuser
Endocarditis in intravenous drug abusers (IVDAs) involves mainly normal valves. Only 20% of the patients have an underlying valvular abnormality when IE is diagnosed. Infection is believed to result from the deposition of bacteria on valves that have sustained microscopic injury through bombardment with drug-associated contaminants injected intravenously. The tricuspid valve is predominantly involved in IVDAs, but aortic and mitral valves may also be damaged. Although S. aureus is known to be the most common causative organism in patients with IE associated with IVDAs, a variety of microorganisms and fungi, including unusual and fastidious organisms (e.g., the HACEK group) and gram-negative organisms (e.g., Pseudomonas species from water sources used), are not uncommon in IVDAs, particularly in the patients who are not meticulous in their injection practices. Polymicrobial infections may also be seen. Because of the higher incidence of right-sided lesions and the generally younger age of the affected group, prognosis for recovery in treated IVDAs’ IE is better than in the general population. However, valvular damage sustained in the course of the infection confers an extremely high risk of recurrent IE in those patients who continue to use intravenous drugs.
Diagnosis of infective endocarditis
Definitive diagnosis of IE requires documentation of sustained bacteremia with a microorganism typical for endocarditis in a patient with an underlying valvular cardiac lesion or by direct demonstration of the pathogen by culture or histopathology of the vegetation or an embolus. The Duke criteria, in use since 1994 to diagnose IE clinically, were primarily developed to facilitate epidemiologic and clinical research. The Duke criteria incorporate echocardiographic findings and IVDA as an important epidemiologic risk factor into the previously formulated Von Reyn criteria for IE and expanded their sensitivity and specificity. Patients with suspected endocarditis were stratified in three categories: definite, possible, and rejected cases. Although its high sensitivity was confirmed by several subsequent studies, some problems emerged with the original criteria with the broad classification of cases as “possible,” the increased relative risk of endocarditis with S. aureus bacteremia, the widespread use of TEE for diagnosis (Figures 37.4 and 37.5), and the classification of culture-negative endocarditis. Modifications of the Duke criteria were proposed in 2000 (Table 37.2) because they have been used as a clinical guide for the diagnosis of IE.
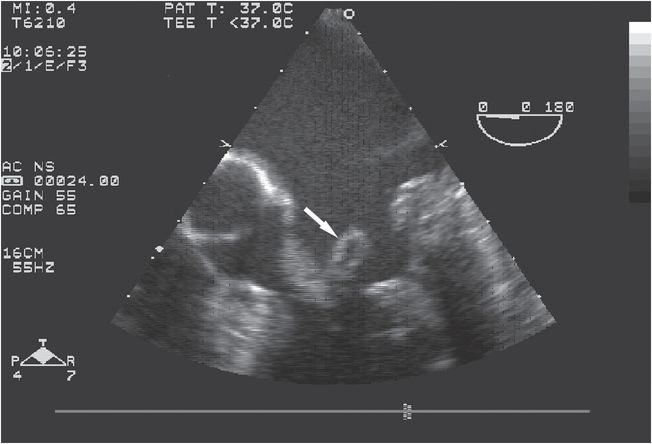
Figure 37.4 Transesophageal echocardiogram of a patient with Haemophilus parainfluenzae infective endocarditis showing large vegetation in mitral valve chordae apparatus (arrow).
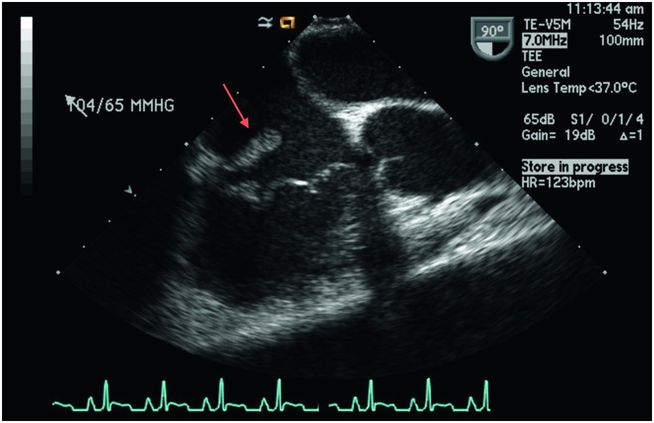
Figure 37.5 Transesophageal echocardiogram of an intravenous drug user with a large vegetation on the tricuspid valve (arrow).
| Definitive infective endocarditis | |
|---|---|
| Pathologic criteria Microorganism demonstrated by culture or histology of vegetation or emboli or intracardiac abscess Histopathologically proven at autopsy or surgery | |
| Clinical criteria 2 major; 1 major and 3 minor criteria; or 5 minor criteria |
| Possible infective endocarditis | |
|---|---|
| 1 major and 1 minor or 3 minor criteriaa |
| Rejected | |
|---|---|
| Alternative diagnosis, resolution of syndrome with ≤4 days of antibiotic; no histopathologic evidence with ≤4 days of antibiotic therapy; does not meet criteria for “possible” IE. |
| Major criteria | Minor criteria |
|---|---|
| Positive blood cultures Typical microorganism for IE from 2 separate blood cultures, as follows: Viridans streptococci, Streptococci bovis, HACEK group, Staphylococcus aureusa, or community-acquired enterococci with no primary focus Persistently positive blood cultures with microorganism consistent with IE: At least 2 positive blood cultures drawn 12 h apart All of 3 or a majority of 4 or more separate blood cultures: first and last sample at least 1 h apart Single blood culture positive for Coxiella burnetii or positive serology: antiphase 1 IgG ab titer 1:800a Endocardial involvement by showing vegetation; or abscess; or new prosthetic valve dehiscence; or de novo valvular regurgitation in echocardiogram. TEE recommended for prosthetic valve, “possible IE” cases, or complicated IE with intracardiac abscessa | Predisposing heart condition or intravenous drug use Fever (38°C) Vascular phenomena Immunologic phenomena Positive blood cultures but short of major criteria or serologic evidence of infection; excludes single positive blood cultures with coagulase-negative staphylococci and organism consistent with IE Echocardiogram consistent with IE but short of major criteria was eliminateda |
Abbreviations: TEE = transesophageal echocardiogram; IE = infective endocarditis; IgG = immunoglobulin G; ab = antibody.
a Modifications from the original Duke Criteria
Therapy
A vegetation consists of microorganisms in high density (108 organisms/g of tissue) and in a reduced metabolic state inside an acellular lesion with impaired host defenses. Eradication of IE is thus almost totally dependent on the efficacy of the antimicrobial therapy. To achieve this end, certain principles of therapy are critical:
In a patient with suspected IE but in whom culture results are not available, empiric antimicrobial therapy should be directed against staphylococci, streptococci, and enterococci unless epidemiologic data point at alternative etiologies. Nafcillin or oxacillin, 2 g IV every 4 hours, and gentamicin, 1 mg/kg IV every 8 hours, may be used as initial therapy. Vancomycin, 15 mg/kg every 12 hours, should be used if the patient is allergic to penicillin. Vancomycin should also be the drug of choice in suspected nosocomial endocarditis because of the high incidence of MRSA and coagulase-negative S. epidermidis (CoNS) in such a setting.
Viridans streptococci and Streptococcus bovis
Antibiotic selection of the therapy of streptococcal endocarditis is based on the minimal inhibitory concentration (MIC) of the isolated organism to penicillin (Table 37.3). Viridans streptococci and S. bovis are generally highly susceptible to penicillin (MIC ≤0.12 µg/mL) and can be treated with aqueous penicillin G or ceftriaxone for 4 weeks. The addition of gentamicin can shorten therapy to 2 weeks, but such therapy should be reserved for patients with normal renal function and uncomplicated (i.e., short duration, minimal distal disease) endocarditis. The same regimen applies to the therapy of streptococcal PVE, but the duration of treatment is prolonged to 6 weeks. For streptococci with moderate resistance to penicillin (MIC 0.12–≤0.5 µg/mL), the addition of gentamicin for the first 2 weeks is always recommended to prevent relapse. IE caused by highly penicillin-resistant viridans streptococci (MIC 0.5 µg/mL) and newly named Abiotrophia defectiva and Granulicatella species (formally known as nutritionally variant streptococci) are difficult to treat and should be treated with the same regimen as recommended for enterococcal endocarditis. Vancomycin is an option only for penicillin-allergic patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





