Breast cancer continues to be the most frequently diagnosed malignancy and the second leading cause of death caused by cancer in women in the United States. Although each of the emerging imaging techniques discussed in this article has advantages compared with standard mammography, they are not perfect, and each has inherent limitations. To date, none have been studied by large randomized clinical trials to match the proven benefits of screening mammography; namely the reduction of mortality caused by breast cancer by nearly 30%.
Key points
- •
Although each of the emerging imaging techniques has advantages compared with standard mammography, they are not perfect, and each has inherent limitations.
- •
To date, no imaging techniques have been studied by large randomized clinical trials to match the proven benefits of screening mammography; namely the reduction of mortality caused by breast cancer by nearly 30%.
- •
More research into breast cancer imaging modalities is required.
Introduction
Breast cancer continues to be the most frequently diagnosed malignancy and the second leading cause of death caused by cancer in women in the United States. Nearly 3 million women were estimated to be living with breast cancer in the United States in 2011. Approximately, 230,000 new cases and 40,000 deaths caused by breast cancer are estimated to occur in 2014. At present, a woman living in the United States has a 1 in 8 (12.3%) risk of developing breast cancer during her lifetime. Today, standard-of-care breast imaging techniques include digital mammography (DM), targeted ultrasonography, and dynamic contrast-enhanced magnetic resonance imaging (MRI).
Mammography has been the mainstay of breast cancer screening programs in the United States and many European countries since the 1990s. Although mammography remains the only imaging modality shown to reduce mortality caused by breast cancer by nearly 30% in multiple large randomized clinical trials, it has several shortcomings. A well-known limitation of mammography is its decreased sensitivity in breasts with predominantly dense parenchyma. Some of the deficiencies of mammography are caused by the depiction of the three-dimensional (3D) breast in a two-dimensional (2D) format. As a result, overlapping tissue may cloak a potential cancer or create a fictitious lesion with features suspicious of a malignancy that is not present. As a result, further unnecessary work-ups and interventions, including needle and excisional biopsies, are performed, incurring both monetary costs and emotional distress to the patient and society. Another important limitation of mammography is the need for breast compression to perform a high-quality study. Breast compression is perhaps the most frequent cause of patient discomfort and anxiety, particularly after recent radiation therapy.
Efforts to overcome these shortcomings of routine mammography have led to the development of newer breast imaging modalities. Apart from targeted ultrasonography, the ubiquitous adjunct to mammography for diagnostic work-up, breast MRI has evolved over the past decade and is now an established modality in breast imaging. Lack of ionizing radiation and high sensitivity when combined with mammography are some of the advantages of MRI. However, lower specificity, requirement of intravenous contrast, high cost, lower patient tolerance, and lack of access have prevented this technique from replacing routine mammograms. With continued improvements in technique, MRI has become established as the modality of choice for specific indications. At present, MRI is most commonly used as a screening supplement to mammography in women at high risk of developing breast cancer. Other common indications for MRI are evaluation of extent of disease and treatment monitoring such as patients receiving neoadjuvant chemotherapy for locally advanced breast cancer. Earlier in the development of this technology, there was prevalent use of MRI for surgical planning, which has now subsided, primarily because of studies with conflicting results regarding desired outcomes such as lowered reexcision and local recurrence rates as well as a paucity of data for survival outcomes. The exact criteria of use beyond screening of high-risk women remains a topic of controversy. Techniques and clinical indications for this modality with an expanding body of evidence for its utility are reviewed elsewhere.
Other breast imaging modalities continue to be developed with hopes of overcoming the challenges of mammography, MRI, and ultrasonography. These modalities include automated whole-breast ultrasonography (AWBUS), digital breast tomosynthesis (DBT), dedicated breast computed tomography (bCT), contrast-enhanced DM (CEDM), and nuclear medicine studies such as positron emission mammography (PEM) and breast-specific gamma imaging (BSGI). These emerging breast imaging technologies show promise in improving the current standards of practice of breast imaging and are the subject of this article.
Introduction
Breast cancer continues to be the most frequently diagnosed malignancy and the second leading cause of death caused by cancer in women in the United States. Nearly 3 million women were estimated to be living with breast cancer in the United States in 2011. Approximately, 230,000 new cases and 40,000 deaths caused by breast cancer are estimated to occur in 2014. At present, a woman living in the United States has a 1 in 8 (12.3%) risk of developing breast cancer during her lifetime. Today, standard-of-care breast imaging techniques include digital mammography (DM), targeted ultrasonography, and dynamic contrast-enhanced magnetic resonance imaging (MRI).
Mammography has been the mainstay of breast cancer screening programs in the United States and many European countries since the 1990s. Although mammography remains the only imaging modality shown to reduce mortality caused by breast cancer by nearly 30% in multiple large randomized clinical trials, it has several shortcomings. A well-known limitation of mammography is its decreased sensitivity in breasts with predominantly dense parenchyma. Some of the deficiencies of mammography are caused by the depiction of the three-dimensional (3D) breast in a two-dimensional (2D) format. As a result, overlapping tissue may cloak a potential cancer or create a fictitious lesion with features suspicious of a malignancy that is not present. As a result, further unnecessary work-ups and interventions, including needle and excisional biopsies, are performed, incurring both monetary costs and emotional distress to the patient and society. Another important limitation of mammography is the need for breast compression to perform a high-quality study. Breast compression is perhaps the most frequent cause of patient discomfort and anxiety, particularly after recent radiation therapy.
Efforts to overcome these shortcomings of routine mammography have led to the development of newer breast imaging modalities. Apart from targeted ultrasonography, the ubiquitous adjunct to mammography for diagnostic work-up, breast MRI has evolved over the past decade and is now an established modality in breast imaging. Lack of ionizing radiation and high sensitivity when combined with mammography are some of the advantages of MRI. However, lower specificity, requirement of intravenous contrast, high cost, lower patient tolerance, and lack of access have prevented this technique from replacing routine mammograms. With continued improvements in technique, MRI has become established as the modality of choice for specific indications. At present, MRI is most commonly used as a screening supplement to mammography in women at high risk of developing breast cancer. Other common indications for MRI are evaluation of extent of disease and treatment monitoring such as patients receiving neoadjuvant chemotherapy for locally advanced breast cancer. Earlier in the development of this technology, there was prevalent use of MRI for surgical planning, which has now subsided, primarily because of studies with conflicting results regarding desired outcomes such as lowered reexcision and local recurrence rates as well as a paucity of data for survival outcomes. The exact criteria of use beyond screening of high-risk women remains a topic of controversy. Techniques and clinical indications for this modality with an expanding body of evidence for its utility are reviewed elsewhere.
Other breast imaging modalities continue to be developed with hopes of overcoming the challenges of mammography, MRI, and ultrasonography. These modalities include automated whole-breast ultrasonography (AWBUS), digital breast tomosynthesis (DBT), dedicated breast computed tomography (bCT), contrast-enhanced DM (CEDM), and nuclear medicine studies such as positron emission mammography (PEM) and breast-specific gamma imaging (BSGI). These emerging breast imaging technologies show promise in improving the current standards of practice of breast imaging and are the subject of this article.
Ultrasonography refinements
Targeted ultrasonography has been a long-standing adjunct to mammography for problem solving and biopsy planning. The advantages of ultrasonography are wide availability, lack of ionizing radiation, and lower cost compared with other techniques such as breast MRI. With increasing awareness of the limitations of mammography, ultrasonography has gained popularity as a supplemental screening tool. Many states such as Texas, New York, Connecticut, and California now have legislation mandating that women be informed of their breast density, encouraging a dialogue between the patient and her referring physician regarding the need for additional screening. Legislation in at least 1 state, Connecticut, has more specific language regarding screening ultrasonography that mandates insurance companies to pay for the examination if recommended by a physician. Whether legislation is the best way to improve clinical outcomes is debatable because there are always unintended consequences resulting from mandates and regulations.
Several studies have shown an incremental cancer detection rate of up to 4.2 cancers per 1000 women screened when ultrasonography is added to mammography screening. The largest trial of screening ultrasonography was performed in 2637 women with increased risk for breast cancer as part of The American College of Radiology Imaging Network (ACRIN) 6666. The addition of a single screening whole-breast ultrasonography to screening mammogram increased the cancer yield from 7.6 to 11.8 per 1000 screened. This gain was balanced by a lower specificity and positive predictive value (PPV) for ultrasonography (8.9%) compared with mammography (22.6%). The call-back rate from the physician-performed examination using hand-held ultrasonography was 5.4% in the ACRIN 6666, which is lower than mammography (5%–12%). The recall rate with screening ultrasonography for additional needed imaging may increase with AWBUS, because the physician is not able to evaluate the findings in real time during scanning. Moreover, ultrasonography is costly in terms of time management, requiring adjustments in the practice workflow and additional qualified personnel. In the ACRIN 6666 trial, along with most other screening ultrasonography studies, a physician using a hand-held device performed the examination. The median time to perform screening breast ultrasonography was 19 minutes, which significantly adds time spent per patient doing a screening study. Similar outcomes have been reported by technologist-performed studies. This labor-intensive and time-consuming protocol has been a major obstacle to widespread implementation of ultrasonography screening.
AWBUS is a recent refinement in technique compared with hand-held ultrasonography that offers some advantages. In AWBUS, the ultrasonography probe is attached to and controlled by a mechanized arm that scrolls over the breast section by section. Physician time is spent on the interpretation of images rather than on acquiring them. Given the automated nature of the examination, dependence on operator skill at the time of acquisition is eliminated. The average examination times are 15 minutes and the technique is limited in large-breasted women, in whom manual scan times are anticipated to take longer. One system approved by the US Food and Drug Administration (FDA) for screening is currently available in the United States. High false-positive rates and low PPV remain obstacles to widespread implementation of AWBUS.
In addition to having a poorer PPV than mammography, there is currently no established Current Procedural Terminology (CPT) code that specifically applies to either method of screening ultrasonography. The performance and interpretation of this lengthier and more laborious screening examination than its more focused targeted counterpart are reimbursed at the same rate using the same CPT codes at this time.
DBT
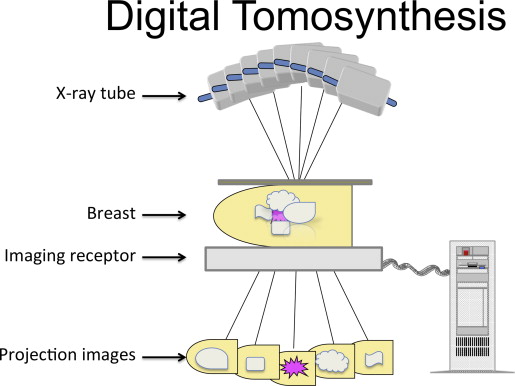
DBT (also referred to as 3D mammography) is a modified mammographic technique designed to increase lesion visibility by decreasing superimposition of adjacent parenchyma. Unlike conventional mammography, in which the x-ray tube and receptor are stationary, producing a single image, in DBT the x-ray source moves through a limited angle range (15°–60°) relative to the receptor and breast in standard projection ( Fig. 1 ). A series of low-dose images are produced through the compressed breast and reconstructed into a volumetric 3D dataset. Images reconstructed at selected thinness ranging from 1 to 10 mm may be viewed individually or as a cine-loop. Separating the breast tissue into a stack of thinly reconstructed images minimizes the effects of tissue overlap. Several manufacturers have developed their own models with variations on the same theme. The tomosynthesis units currently in clinical use have the capability of obtaining 2D DM and tomosynthesis views of the breast during the same compression in standard craniocaudal (CC) and mediolateral oblique (MLO) projections. In 2011, the FDA approved this DM/DBT combination (Selenia Dimensions, Hologic) for clinical practice in the United States.
Early experimental clinical studies have shown an increase in screening sensitivity, decrease in false-positives, and improved performance of DM in combination with DBT compared with DM alone. Several small studies of lesions including masses and microcalcifications showed physician preference for tomosynthesis image quality compared with DM. Interim results from several ongoing trials have shown the potential for DBT to decrease false-positive recall rates with equal or improved cancer detection rates to DM. The Oslo Tomosynthesis Screening Trial is one of the largest prospective single-center tomosynthesis studies performed to date. Skaane and colleagues reported initial results for 12,631 women, aged 50 to 70 years, who were invited for biennial screening comparing standard 2-view DM (2v DM) alone with combined 2v DM and 2-view DBT (Selenia Dimensions, Hologic). They reported a significant 27% increase in cancer detection (8.0 vs 6.1 per 1000 examinations) for DM plus DBT compared with DM alone. False-positive rates decreased from 61.1 to 53.1 per 1000 patients screened with DM alone compared with DM plus DBT; a decrease of 15% in screening recall rates. Similar results were reported by the Screening with Tomosynthesis or standard Mammography (STORM) trial, a prospective Italian screening study of women 48 years of age or older. Eight radiologists interpreted the 2v DM (Selenia Dimensions, Hologic) screening study followed by the 2v DM plus DBT in 7294 subjects. An incremental cancer detection rate of 2.7 cancers per 1000 patients (33.9%) attributable to an integrated DM and DBT screening approach relative to 2v DM alone was reported. The decrease in the false-positive recall rate of 2% based on DM alone to 1% with the addition of DBT supports the results of previous studies. The latest study in the United States retrospectively evaluated 454,850 screening examinations from 13 sites interpreted by 139 radiologists. The evaluation included 281,187 (61.8%) DM performed in the year before implementation of tomosynthesis and 173,663 (38.2%) DM plus DBT in the year following. The cancer detection rate increased from 4.2 per 1000 patients screened with DM to 5.4 per 1000 patients screened with DM plus DBT. The investigators reported a model-adjusted false-positive rate per 1000 screens of 107 with DM versus 91 for mammography combined with tomosynthesis. These studies add to a growing body of evidence that DBT in combination with DM reduces false-positive examinations and improves cancer detection rates.
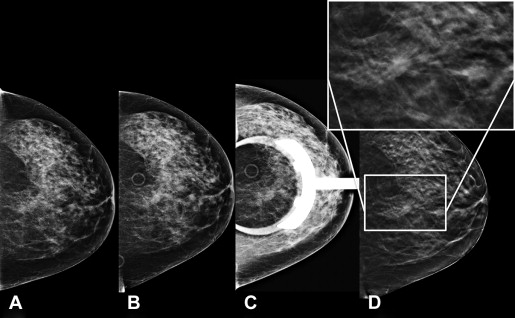
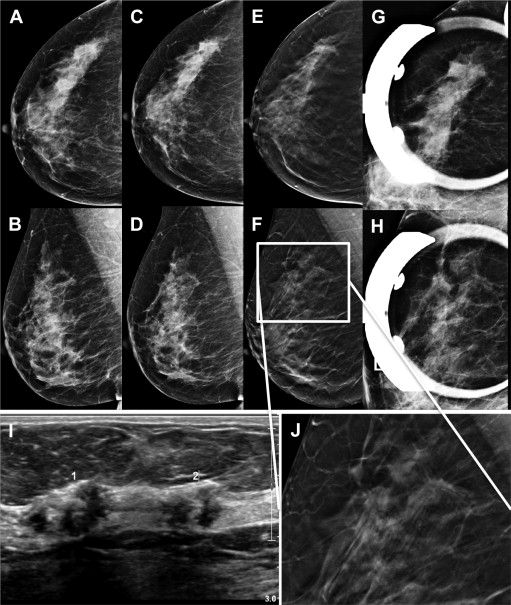
Several studies have suggested that there may be a role for DBT in standard diagnostic work-up of symptomatic patients and screening callback examinations ( Fig. 2 ). In a reader study of 67 subjects with 30 malignant and 37 benign breast masses, Noroozian and colleagues compared DBT with standard mammographic spot compression views. They reported no significant difference in accuracy but slightly better mass visibility with DBT. Tagliafico and colleagues showed no statistical difference between DM spot compression views and DBT in a small prospective study of 52 screening recalls of noncalcified lesions. However, lesion conspicuity was better for DBT (4.1) than for spot compression views (2.9). A more recent retrospective analysis of 217 noncalcified lesions was designed to compare the diagnostic performance of DBT and DM for 8 readers. Receiver operating characteristic curves offer a way to measure the trade-off between sensitivity and specificity for a diagnostic test. The area under the curve (AUC) is a metric of better performance as it approaches a value of 1.00. Using this analysis, tomosynthesis (AUC, 0.87) significantly outperformed conventional supplemental diagnostic views (AUC, 0.83) in its performance measuring probability of malignancy. Additional studies have also suggested the potential for DBT to replace standard mammographic diagnostic views ( Fig. 3 ).