Interventional oncology, a term commonly used to indicate the minimally invasive procedures performed by interventional radiologists to diagnose and manage cancer, encompasses a broad spectrum of techniques unique to interventional radiology that have been established as a vital part of the multidisciplinary oncologic cancer care team. This article provides an updated overview of the variety of applications of image-guided procedures to distinct clinical scenarios, such as the diagnosis, treatment, and management of complications of malignancies.
Key points
- •
Interventional radiology provides a wide range of minimally invasive procedures that play a critical role on the diagnosis, treatment, and palliation of patients with cancer.
- •
Percutaneous image-guided biopsy is an established method for obtaining tissue specimens with high diagnostic accuracy, few complications, and lower costs when compared with more invasive procedures.
- •
Percutaneous ablation can be used as an alternative to surgical resection in patients with primary and secondary malignancies with similar outcomes in selected cases.
- •
Image-guided procedures can be used in the management of the complications of malignancy, improving a patient’s quality of life.
Introduction
In 1964, Dr Charles Dotter, using a combination of basic guidewires and catheters, successfully dilated a focal stenosis of the superficial femoral artery in a patient with painful leg ischemia and gangrene who had refused surgery, thereby reestablishing flow and saving her limb. The success, and symbolism, of this procedure opened the way for a shift in a long-last paradigm in medicine: the use of medical imaging solely as a diagnostic tool. Over the subsequent 3 decades, the investigational mind and talent of many interventional radiologists and the unparalleled technological advances in medical imaging and material development were pivotal for the establishment of interventional radiology as a distinct medical specialty.
Interventional oncology, a term commonly used to refer to minimally invasive procedures performed by interventional radiologists for diagnosing and treating cancer, accounts for a broad spectrum of procedures unique to interventional radiology that have demonstrated unequivocal clinical benefits and established their roles as an integral part of the multidisciplinary oncologic cancer care team. This article provides an updated overview of the role of image-guided interventions in oncology.
Introduction
In 1964, Dr Charles Dotter, using a combination of basic guidewires and catheters, successfully dilated a focal stenosis of the superficial femoral artery in a patient with painful leg ischemia and gangrene who had refused surgery, thereby reestablishing flow and saving her limb. The success, and symbolism, of this procedure opened the way for a shift in a long-last paradigm in medicine: the use of medical imaging solely as a diagnostic tool. Over the subsequent 3 decades, the investigational mind and talent of many interventional radiologists and the unparalleled technological advances in medical imaging and material development were pivotal for the establishment of interventional radiology as a distinct medical specialty.
Interventional oncology, a term commonly used to refer to minimally invasive procedures performed by interventional radiologists for diagnosing and treating cancer, accounts for a broad spectrum of procedures unique to interventional radiology that have demonstrated unequivocal clinical benefits and established their roles as an integral part of the multidisciplinary oncologic cancer care team. This article provides an updated overview of the role of image-guided interventions in oncology.
Clinical use of image-guided interventions
Cancer Diagnosis
The advances in morphologic and functional imaging of the past decade, along with an expanding accessibility and use of this technology, have led to dramatic improvements in diagnosing and monitoring cancer. Nevertheless, accurate diagnosis invariably relies on obtaining adequate pathologic specimens. More recent understanding of molecular biology and the use of molecularly targeted agents in the cancer armamentarium have directed cancer treatment to targeted therapy. In this new horizon, the requirements for obtaining tumor tissue for diagnosis not only play a role in the diagnosis of the malignancy but also could potentially provide useful information that could tailor patient therapy, ultimately providing objective clinical response in selected patients. Among the modalities for obtaining tumor tissue for histologic diagnosis, percutaneous image-guided biopsy (PIB) is a safe, well-established, and widely used method for obtaining tissue specimens with high diagnostic yield and few complications, making it the alternative for the vast majority of biopsies. PIB also reduces hospital length of stay, costs associated with biopsy, and patients’ anxiety associated with a major surgical intervention.
Various imaging modalities are used to guide percutaneous biopsies. These include fluoroscopy, ultrasonography, computed tomography (CT), CT fluoroscopy, magnetic resonance imaging, positron emission tomography-CT, and combinations of these modalities. The choice of imaging modality is based on the biopsy site, operation preference, potential access routes, ability to visualize the lesion, and the availability and cost of the equipment. Most interventional radiologists prefer the coaxial technique for PIB. This technique involves the initial placement of a thin-walled needle in or close to the target lesion and the advancement of fine-needle aspiration and cutting needles through the thin-walled needle to obtain tissue samples, which allows multiple tissue samples to be obtained without the need for additional passes through the overlying structures and thereby minimizing patient discomfort and complications. This technique also allows continuous access to the target lesion while the initial samples are being analyzed by the cytopathologist, creating an easy path for obtaining new specimens and thus potentially increasing the diagnostic yield ( Fig. 1 ). Fine-needle aspiration uses thin-walled 20- to 25-gauge needles that provide specimens suitable for cytologic and microhistologic evaluations with minimal risk of complications compared with core-needle biopsies. Cutting needles (or core needles) provide core specimens for histologic evaluation and are available in various calibers. Modern small-caliber (18- to 20-gauge) cutting needles consistently provide high-quality specimens without increasing complication rates. With this technique, the success rate of PIB is 70% to 95%, depending on the mix of organ systems, lesion size, and lesion location and the relative proportion of benign versus malignant lesions that are sampled. Further understanding of the sample adequacy for PIB intended for molecular testing and genetic analysis is a subject of current investigations.
Portal Vein Embolization
The incidence of primary liver cancer has continued to rise over the past decade in the United States, and liver metastasis is still frequent. In patients with primary tumors and metastases confined to the liver, liver resection remains the mainstay of curative therapy. Despite technical advances in surgical technique and the care of postoperative patients, complications, such as fluid retention, cholestasis, and impaired synthetic function, still prolong recovery and hospital stays in patients who undergo hepatic resection. Among the elements associated with postoperative liver insufficiency, volume of the future liver remnant (FLR) is a strong and independent predictor of postsurgical hepatic dysfunction and complications. The use of portal vein embolization (PVE) relies on the rationale of promoting preoperative increase in FLR by the hypertrophy caused by the redirection of blood flow to nonembolized liver segments, with the intent of reducing the incidence of postoperative liver insufficiency and increasing the number of patients eligible for major hepatic resection.
The workup for PVE should begin with the calculation of total estimated liver volume (TELV) and the acquisition of volumetric CT images to calculate FLR volume. Among the ways of calculating TELV, some prefer the formula proposed by Vauthey and colleagues, which was derived from the close relationship between body surface area and TELV (TELV = −794.41 + 1267.28 × body surface area). The FLR-TELV ratio is used as the standardized (preoperative) FLR that will be correlated with the surgical outcome. The FLR volume required to minimize the risk of hepatic failure after surgery is 20% and 30% in patients without cirrhosis who did and did not receive systemic chemotherapy, respectively, and 40% in patients with cirrhosis. Patients with an FLR volume below the appropriate threshold should be considered for PVE, considering individual factors on a case-by-case basis. Contraindications for PVE include overt clinical portal hypertension, extensive portal vein tumor invasion, and complete lobar portal vein occlusion in view that the portal vein flow is already diverted in this situation. Relative contraindications include partial tumor invasion of the portal vein, extrahepatic metastasis, tumor site precluding safe access to the portal vein, renal failure, and mild portal hypertension.
PVE is classically performed via 1 of the 3 different approaches: the percutaneous transhepatic, the percutaneous transjugular, or the intraoperative transileocolic venous approach. The most commonly used approach worldwide, the percutaneous transhepatic approach, can be categorized as contralateral or ipsilateral; the latter is gaining favor because it reduces the risk of injuring the FLR compared with the contralateral access ( Fig. 2 ). A series of conventional angiographic catheters and guidewires are passed through the percutaneous vascular access to the portal vein system to perform portal vein venography, embolize the hepatic segments to be resected, and measure preembolization and postembolization pressures. Among the embolic materials preferred for PVE, we give preference to small (100-μm to 500-μm) calibrated particles and embolization coils within the secondary portal branches to further reduce the portal inflow that could lead to recanalization. Patients who undergo PVE are discharged on the same day. A CT follow-up of 4 to 6 weeks is requested to assess the degree of hypertrophy of the FLR.
PVE provides high technical and clinical success rates, as indicated by hypertrophy of the FLR ranging from 28% to 46%. In a recent systematic review of 44 articles that included 1791 patients who underwent embolization, van Lienden and colleagues calculated a mean hypertrophy rate of 37.9% ± 0.1%, a success rate of 99.3%, and a mortality rate of 0.1%. In a recent study of 358 patients from our institution, the median pre-PVE and post-PVE FLR volumes were 19.5% and 29.7%, respectively, and the rates of postoperative hepatic insufficiency and 90-day mortality were 8.3% and 3.8%, respectively.
Cancer Treatment
Transarterial hepatic treatments
Transarterial interventional techniques used for palliation or bridge therapy of primary and secondary malignant hepatic tumors emerged due to the peculiarities of blood flow to primary and several secondary hepatic malignancies, which are preferentially supplied via the hepatic artery. Among these techniques, the most common are bland embolization of the arterial supply to the tumor to induce ischemia and tumor necrosis, regional intra-arterial infusion of cytotoxic or immunotherapeutic agents to enhance the tumoricidal effect, the combination of those techniques (chemoembolization), and transarterial delivery of yttrium-90 ( 90 Y)-coated microspheres (radioembolization).
For transarterial chemoembolization (TACE), a chemotherapeutic drug or drugs are added to the embolic agent, on the basis of the theory that tumor ischemia caused by arterial embolization has a synergistic effect with the chemotherapeutic drugs. Several chemotherapeutic agents, most commonly doxorubicin and cisplatin, can be mixed with one or more embolic agents. Recently, the development of calibrated microparticles (DEB-TACE) has gained wide acceptance ( Fig. 3 ). These drug-eluting microspheres allow more reliable distal occlusion of small vessels and delivery of high-dose chemotherapy to the tumor with a very low systemic circulation of the chemotherapeutic agent, thus reducing the associated systemic adverse effects when compared with other traditional forms of TACE. Transarterial radioembolization (TARE) consists of the delivery of microspheres loaded with yttrium-90 ( 90 Y), a pure beta emitter. Like other transarterial therapies, 90 Y-loaded microspheres can deliver beta radiation preferentially to tumors following hepatic artery delivery via embolization of tumor-related arterioles. This approach potentially creates an intense local radiotherapeutic effect that is proportional to the density of the microsphere distribution. Compared with nonselective extracorporeal x-ray radiotherapy, TARE allows the particles to be deposited predominantly within the tumor vasculature, thereby leading to tumor damage while preserving the surrounding liver parenchyma. This feature allows the delivery of substantially higher radiation doses than those that can be safely delivered via external-beam radiotherapy.
TACE and TARE can be used as palliative treatment for patients who are not candidates for surgical resection or liver transplantation. They also can be used as neoadjuvant therapy with either the intent to prevent tumor progression or as a downstaging strategy for patients with a tumor burden beyond the acceptable inclusion criteria for liver transplantation. For patients with hepatocellular carcinoma (HCC) classified by the Barcelona Clinic Liver Cancer (BCLC) guidelines as intermediate stage (BCLC-B), transarterial chemoembolization is considered the standard of care. In a recent study, a median survival of 42.8 months was achieved with the use of DEB-TACE loaded with doxorubicin in patients with BCLC-B. For patients with unresectable metastatic colorectal disease to the liver, results of a recent phase I pilot study and the final results of a phase III study, respectively, demonstrated that DEB-TACE loaded with irinotecan increased the 6-month overall response rate of hepatic lesions when compared with first-line systemic chemotherapy and increased overall survival, response rate, progression-free survival, and quality of life compared with second-line therapy. TARE has been investigated with patients with intermediate-stage or advanced-stage HCC. In a recent phase II trial, 17 patients with intermediate-stage HCC who did not have portal vein thrombosis were treated with TARE; disease control was achieved in 15 patients, time to progression was 13 months, and overall survival duration was 18 months (range, 12–38 months). In a multicenter trial that assessed the use of radioembolization with 90 Y for patients with HCC, the 87 patients with BCLC-B HCC who were treated with 90 Y had a median survival of 16.9 months. The result of the trial suggested that radioembolization with 90 Y may be particularly promising for patients with intermediate-stage HCC who are poor candidates for TACE as well for patients for whom prior TACE or bland embolization is ineffective, highlighting the possibility of using TARE as a complement to TACE.
Nonhepatic transarterial treatment
Intra-arterial procedures can be used to manage primary or secondary cancers, such as renal cell carcinoma (RCC) and pelvic and bone malignancies. These procedures can be used as a palliative stand-alone method, in combination with chemotherapy, ablative or surgical therapies, or as preoperative adjunct to facilitate surgical excision, reducing intraoperative blood loss and postoperative complications.
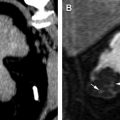
Renal artery embolization in the setting of RCC has been widely used since its introduction as a preoperative and palliative strategy. Preoperative embolization can facilitate surgical excision by collapsing the tortuous veins covering the surface of the tumor and the renal hilum that can impede access to the renal artery during surgery. This technique also can reduce the size and extension of a tumor thrombus invading the renal vein or inferior vena cava, and it can reduce intraoperative blood loss and, although controversial, the operating time. In general, nephrectomy is performed within 24 to 72 hours after embolization to take maximum advantage of the infarction-induced edematous rim around the kidney and to reduce distress from postembolization syndrome. More recently, renal artery embolization has been used before thermal ablation for patients with RCC with the objective of reducing the heat-sink effect created by the vessels in the tumoral bed during ablation and potentially enhancing treatment efficacy by increasing the thermal ablated volume ( Fig. 4 ). Similarly, superselective tumor embolization has been used as an adjunct strategy to facilitate laparoscopic removal of tumors with a nephrometry score of 6 or higher to reduce blood loss and operating time while maintaining 5-year local recurrence rates similar to patients who undergo open partial nephrectomy. Palliative embolization of RCC has been used with varying degrees of success for patients with unresectable RCC who are not candidates for surgical resection to control tumor-related symptoms, such as flank pain, hemorrhage, congestive heart failure due to large arteriovenous fistulas, hypercalcemia, hypertension, and polycythemia. A review of all studies on arterial embolization for RCC published between 1973 and 1997 showed that only a small group of patients with massive hematuria, flank pain, or paraneoplastic syndrome may benefit from palliative embolization. More recently, small case-series demonstrated successful control of hematuria and flank pain after renal embolization. The effectiveness of this procedure in symptom palliation awaits further study and validation.
Regional intra-arterial chemotherapy and embolization can be used to treat pelvic malignancies. Intra-arterial chemotherapy, especially in combination with radical surgery, is useful in managing advanced uterine cervical carcinoma. In a study of 25 patients with stage IIB to IIIB uterine cervical carcinoma treated with pelvic intra-arterial cisplatin and bleomycin followed by surgery or radiotherapy, a clinical response rate of 80% and an operability rate of 72% was achieved. Pelvic intra-arterial chemotherapy also can be followed by uterine artery embolization to enhance the local therapeutic effect. The use of intra-arterial cisplatin administration in combination with radiotherapy for patients with muscle-invasive urinary bladder cancer has been investigated; the reported complete response rate ranged from 74% to 90%, the bladder preservation rate ranged from 75% to 100%, and the survival rate was similar to that achieved with cystectomy.
Transcatheter arterial embolization for hypervascular primary and secondary bone tumors is generally performed preoperatively or for palliation. Hypervascular bone metastases, which are seen in 30% to 45% of patients with RCC and thyroid carcinoma, are by far the most commonly embolized bone metastases. Owing to the hypervascular nature of these lesions, surgical intervention can be complicated by excessive, uncontrollable bleeding. The use of preoperative arterial embolization has been reported for bone metastases from renal, thyroid, breast, and lung malignancies and from melanoma, and has been shown to reduce intraoperative blood loss. Embolization also is used to inhibit tumor growth and to reduce pain or bleeding in patients with unresectable tumors.
Percutaneous ablation therapy
Percutaneous therapy refers to the use of nonthermal and thermal percutaneous ablative technologies to treat cancer under imaging guidance. With this method, the target lesion is treated by the insertion of a needle or probe (or both) with subsequent delivery of energy or substance (or both) by imaging guidance. This technique is widely used for primary and metastatic tumors of the liver, lung, kidney, adrenal glands, and soft tissue. Nonthermal technology includes chemical ablation with ethanol or acetic acid instillation and irreversible electroporation. Thermal ablative technology includes radiofrequency ablation (RFA), cryoablation, and microwave ablation.
Percutaneous ethanol injection is the seminal percutaneous ablation technique. With a guiding needle, absolute ethanol is injected inside the tumor and around it, inducing coagulative necrosis as a result of cell dehydration, protein denaturation, and chemical occlusion of small vessels. This method is a well-established technique for treating nodular type HCCs. Although percutaneous ethanol injection is widely available and efficacious, the extent of necrosis obtained by this method is correlated with the size of the lesions: the complete response rate is higher in smaller HCCs than in large HCCs (90%, 70%, and 50% of tumors measuring <2, 2–3, and 3–5 cm, respectively). Irreversible electroporation induces cell death by disrupting the electrical potential across cell membranes by creating permanent nanopores through the plasma membrane, which affects cell homeostasis. Irreversible electroporation appears to be free from the heat-sink effect that affects other thermal ablative technologies and is safe for use with hepatic tumors adjacent to hepatic veins and portal pedicles.
RFA involves delivering an alternating electrical current with a probe placed directly into the tumor. The resulting frictional heat and movement of electrons within the lesion and surrounding tissues generates heat in the immediate vicinity of the electrode. This heat is then conducted to the surrounding environment and results in coagulative necrosis of a finite volume of tissue. This technology has become the first-line modality for percutaneous hepatic ablation for patients with HCC and metastatic disease to the liver, mainly because it yields complete necrosis with fewer sessions than is required for percutaneous ethanol injection, thus leading to better local disease control. The size and shape of the ablation zone varies depending on the amount of energy applied, type and number of electrodes, duration of ablation, and inherent tissue characteristics. Because of its efficacy and safety profile, RFA is now offered to patients who are surgical candidates with comparable 5-year survival outcomes from resection. The limitations of the technique include the heat-sink effect, whereby adjacent blood vessels produce perfusion-mediated attenuation of thermal energy deposition, potentially leading to incomplete ablation; large lesions (>5 cm); and proximity to thermally sensitive structures, such as the intestinal wall, gallbladder, diaphragm, and nerves, which can be damaged during the course of ablation.
Microwave (MW) ablation promotes tissue heating by causing polar water molecules to continuously realign with an oscillating electromagnetic field that emanates from the microwave antenna placed within the tumor. The continuous realignment creates heat by agitating water molecules in the surrounding tissue, thereby producing friction and heat and inducing cellular destruction via coagulative necrosis. In contrast to RFA, microwave propagation is not affected by low electrical conductivity, high tissue impedance, or low thermal conductivity. Compared with other available ablative technologies, MW creates larger tumor ablation volumes with consistently higher intratumoral temperatures, has faster ablation times, and an improved and a more favorable convection profile, thus resulting in a reduction in the heat-sink effect created by vessels near to the ablated zone. Recent improvements in MW engineering, along with expanded application of and experience with this technology, could create more effective ablation zones than RFA does ( Fig. 5 ).