Effect of Aging on Gastrointestinal Function: Introduction
The aging process has clinically significant effects on oropharyngeal and upper esophageal motility, colonic function, gastrointestinal (GI) immunity, and GI drug metabolism (Figure 89-1). On the other hand, because the GI tract exhibits considerable reserve capacity, many essential aspects of GI function, such as intestinal secretion, are preserved with aging. Despite such adaptation, superimposed effects of chronic diseases and environmental/lifestyle exposures (medications, alcohol, tobacco) can further impair GI function in older patients. A modest decline in gastric mucosal cytoprotection or esophageal acid clearance may become significant when superimposed side effects of certain medications or concurrent disease are also present. Certain age-related changes in GI function, such as constipation, are viewed as dysfunctional by patients and health care providers. Research areas that have been identified as important in aging include the pathophysiology of swallowing disorders, esophageal reflux, dysmotility syndromes, GI immunobiology, and the cellular mechanisms of neoplasia in the GI tract. Animal studies provide important insights into the cellular physiology of aging, despite the issue of species variation.
Figure 89-1.
Effects of physiological aging on the gastrointestinal tract. This schematic diagram summarizes significant effects of aging on various divisions of the gastrointestinal tract. Key: up arrow, increased, down arrow, decreased. (Revised from Hall and Wiley. Age-associated changes in gastrointestinal function. In: Hazzard et al., eds. Principles of Geriatric Medicine and Gerontology, 5th ed. New York, New York: McGraw-Hill; 2003 [chapter 63].)
Oropharyngeal Function
The increasing likelihood of dental decay and tooth loss with aging affects the efficiency and completeness of mastication (also see Chapter 42). Chewing and swallowing are impaired by xerostomia, which affects roughly 25% of older patients, while as many as 50% have subjective complaints of dry mouth. Medication side effects are a common cause of xerostomia, while a minority is caused by specific diseases affecting the salivary glands, such as Sjogren’s syndrome. A mild loss of saliva production appears to occur with normal aging.
After food is broken up in the mouth by mastication, the act of swallowing moves the food bolus from the oral cavity into the pharynx and esophagus (also see Chapter 41). The oral and pharyngeal stages of swallowing are regulated by cortical input to medullary swallowing centers, which innervate skeletal muscle groups in the pharynx. The proximal esophagus contains skeletal muscle controlled by nerves from the medullary swallowing centers, whereas the mid- and distal esophagus consists of smooth muscle regulated by intrinsic enteric innervation and extrinsic innervation by the vagus nerve.
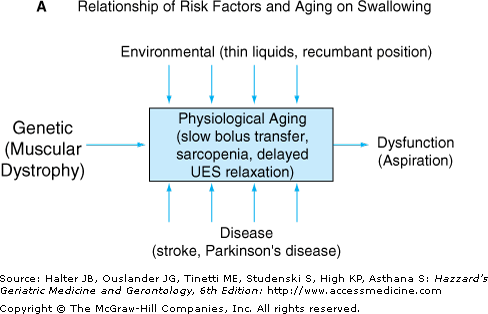
Oropharyngeal swallowing disorders are most commonly observed in patients with cognitive and/or perceptual dysfunction secondary to stroke or dementia, or chronic neurodegenerative diseases that affect the brainstem or motor neurons, such as Parkinson’s disease, myasthenia gravis, or amyotrophic lateral sclerosis. Normal aging, however, is associated with alterations that predispose older individuals to dysphagia. Video fluoroscopy demonstrates abnormal transfer of a food bolus from the oral cavity to the pharynx in up to 60% of elderly patients without dysphagia. Other anatomic changes in esophageal neuromuscular anatomy occur in the aged orpharynx, such as cricopharyngeal bars caused by osteoarthritic spine disease. The pathophysiological significance of these are not clear, however, as they are seen in older patients with normal deglutition. Upper esophageal sphincter pressure gradually decreases with age, and is associated with a delay in relaxation after deglutition. Aspiration risk is increased in older subjects by delayed elevation of the larynx and decreased ability to clear food from the pharynx compared to younger subjects. However, clinically significant aspiration usually does not occur unless there are superimposed risk factors such as neurodegenerative diseases (e.g., Parkinson’s disease) or medications that affect neuromuscular function. Figure 89-2 illustrates how various risk factors may interact with aging effects to lead to clinical swallowing problems, and is a model that may apply to how aging contributes to other GI tract disorders as well.
Figure 89-2.
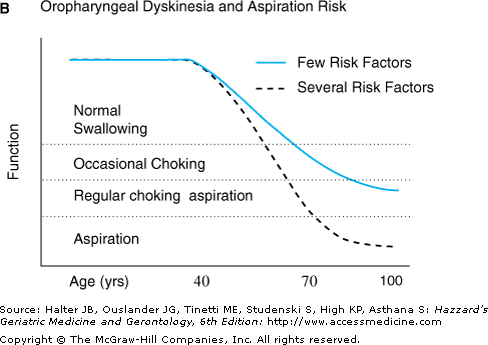
(A) Physiological aging maybe thought of as a baseline state upon which can be superimposed the effects of risk factors such as genetic predisposition, environmental exposure, and superimposed disease. The net result is symptomatic dysfunction in senescence that results in impairment. An example of this kind of synergy is dysphagia, which can manifest as a result of a combination of superimposed risks such as neurodegenerative disease and drug-induced damage on age-related oropharyngeal dyskinesia. UES, upper esophageal sphincter. (B) Graph illustrating how risk factors may affect swallowing function at various ages. Horizontal solid lines indicate three theoretical thresholds for clinically significant dysphagia (mild, moderate, severe symptom). Physiological aging of the gastrointestinal tract in individuals with few risk factors for dysphagia (solid blue line) results in minimal alterations in swallowing in middle age through the sixth decade. Superimposed risk factors such as neuromuscular disease (dashed line) can lead to earlier onset of dysphagia, and increased risk of moderate or severe dysphagia.
Esophageal Function
Age-associated changes in the anatomy of the esophageal body appear to be minimal. Thickness of esophageal smooth muscle and amplitude of peristaltic contractions appear unaffected by aging. Although the number of myenteric neurons in the esophagus decreases with age, the functional significance is unclear. While some studies have reported impaired relaxation or decreased contractions of the lower esophageal sphincter (LES) with aging, more recent reports suggest that concomitant disease accounts for altered resting pressure of the aged LES.
Complaints of dysphagia, regurgitation, chest pain, and heartburn are fairly common in the geriatric population, with a prevalence of 35% reported in the general population aged 50 to 79 years (see Chapter 91). However, it is difficult to demonstrate a consistent relationship between symptoms and underlying pathophysiology, as demonstrable esophageal abnormalities are found in only 20% to 30% of symptomatic patients. A particular concern in an elderly patient is that symptoms of dysphagia are more likely to have a serious underlying etiology, such as malignancy. Elderly patients also appear to be more susceptible to complications of inadequately treated chronic esophageal disease, such as aspiration, malnutrition, and Barrett’s metaplasia. Attempts at symptom analysis to identify subgroups at risk for serious underlying disease have met with mixed success.
The term “presbyesophagus” was coined to describe age-related changes in esophageal function, including decreased contractile amplitude, polyphasic waves in the esophageal body, incomplete relaxation of the lower esophageal sphincter, and esophageal dilation. The most common alteration in motility observed in older subjects was an increase in rapidity of propagation of the peristaltic wave, together with increased simultaneous contractions. Esophageal dysmotility in older patients is more often a result of diabetes mellitus and neurological disorders, or the side effects of medications, rather than aging per se.
Symptoms related to gastroesophageal reflux disease (GERD) are a frequent complaint of elderly patients (see Chapter 91). Risk factors for reflux-induced esophageal damage include decreased esophageal clearance, gastric factors such as decreased emptying or increased gastric pressure, decreased LES pressure, inappropriate LES relaxation, or the presence of a hiatal hernia, and increased gastric acid secretion. Older patients appear to have an increase in esophageal acid exposure and longer duration of reflux episodes. This maybe caused by decreased occurrence of secondary esophageal peristalsis, an important clearance mechanism for refluxed acid. Other factors, such as gastric emptying and frequency of LES relaxation, are relatively unchanged with aging. Concurrent disease and side effects of medications may play an important role in the pathophysiology of GERD in older patients.
Gastric Function
Decreased clearance of liquids from the stomach has been documented in older patients, and can be exacerbated by anticholinergic medications. Aging is also associated with decreased perception of gastric distension as measured by subjective fullness during inflation of a gastric barostat balloon. Delayed emptying may prolong the gastric contact time of noxious agents, such as nonsteroidal anti-inflammatory drugs (NSAIDs). This may have serious consequences, as significant age-related changes do occur in gastric mucosal defense mechanisms. These may predispose aged people to gastric mucosal injury and subsequent complications of ulceration. Increased risk of gastric injury does not appear to be to the result of excessive secretion of agents that promote mucosal injury, as most healthy older individuals have decreased, not increased, acid and pepsin output. A small minority has severe acid hyposecretion (achlorhydria) associated with atrophic gastritis. Animal studies have shown a small age-related reduction in gastric acid secretion. Gastrin levels tend to rise with age in humans, possibly as a compensatory response to decreased acid production as a result of increased gastric autoantibodies and Helicobacter pylori colonization. The increased prevalence of H. pylori carriage in the elderly population does not appear to result in increased rates of duodenal ulceration, but is associated with increased risk of pernicious anemia and gastric lymphoma.
Increased susceptibility to gastric mucosal injury from NSAIDs in elderly people appears to be caused by a reduction in gastric mucosal cytoprotective factors such as mucosal prostaglandins. Human and animal aging is associated with a significant decrease in gastric bicarbonate, sodium ion and nonparietal fluid secretion, and thinning of the mucus gel layer, particularly in H. pylori-positive individuals. Aging is also associated with a decrease in the basal and injury-induced rate of proliferation of stem cells in the neck of gastric glands in rats. In vivo expression and activity of several intestinal growth factors, such as transforming growth factor alpha (TGFα) and epithelial growth factor (EGF), is decreased in animal models of aging, However, activation of EGFR by membrane-bound TGF-alpha in vitro is actually increased in gastric and colonic mucosa of aged rats, suggesting that the relative lack of activity may not be caused by intrinsic inactivation.
Mucosal blood flow plays an essential role in maintaining gastric mucosal integrity. In rats, aging is associated with a decrease in basal gastric blood flow associated with impaired healing of acid-induced mucosal lesions. Impaired healing of gastric ulcers has also been associated with underlying impairment in sensory neuron function in several aging models.
Small Intestinal Function
The small intestine has a large functional reserve capacity, because of the substantial mucosal surface area available for secretion and absorption. Intestinal surface area does not change after 6 weeks of age in rats. It is unclear whether the specific activity of intestinal disaccharidases and amino-peptidases is affected by aging, as both higher and lower activity has been reported in aged animals compared to youthful controls. Delayed maturation and expression of brush-border enzymes in small intestinal villous epithelial cells during crypt-to-villous migration appears to be the underlying cause. However, absorption of lactose, mannitol, and lipid in individuals greater than 60 years of age is unaffected. Vitamin D absorption and sensitivity is significantly impaired in aged rats. This is also a concern in human studies, which have documented decreased uptake of vitamin D, folic acid, vitamin B-12, calcium, copper, zinc, fatty acids, and cholesterol in aged subjects. Current recommendations by the U.S. Department of Agriculture to increase calcium, B-12, and Vitamin D supplementation in persons older than 70 years of age reflect this new information. Iron is one nutrient that is usually present in overabundance in the diet for older individuals. Older patients maybe at risk of diseases such as hepatic iron overload (particularly in individuals who are monozygotic for hemochromatosis), and iron overload has been implicated in some models of oxidative stress. Older men and postmenopausal women do not require additional iron unless there is superimposed blood loss or bone marrow impairment.