The plasma lipoproteins and the lipids that they transport play a central role in the development of, or protection from, atherosclerosis starting in childhood. The lipoproteins can be usefully divided into the apolipoprotein B (apoB)-containing lipoproteins and the apoA-I-containing lipoproteins. The apoB-containing lipoproteins include chylomicrons and very low density lipoproteins (VLDL) and their remnants, intermediate-density lipoproteins (IDL), low-density lipoproteins (LDL), and Lp(a) [lipoprotein(a)]. For each molecule of an apoB-containing lipoprotein, there is one molecule of apoB. The apoA-I-containing lipoproteins include high-density lipoproteins (HDL) and their subfractions HDL2 and HDL3. ApoA-I constitutes about 70% of the protein on HDL. There are usually three to four molecules of apoA-I for each HDL particle.
Most of the cholesterol in plasma is transported by LDL (60% to 70%) with the remainder carried on HDL (20% to 30%) and VLDL (10% to 15%). Triglycerides (TG) are transported primarily by chylomicrons, VLDL, and their remnants. Increased levels of the apoB-containing lipoproteins promote atherosclerosis and CVD, while low levels of these lipoproteins are associated with reduced CVD and longevity. In distinct contrast, high levels of HDL are usually associated with reduced CVD, while low levels of HDL often promote atherosclerosis and CVD.
Lipoprotein Structure and Function and Transport Pathways
A detailed summary of lipoprotein structure and function may be found in
Chapters 1 and
2. As well, each of the three lipoprotein pathways, exogenous lipid transport, endogenous lipid transport, and reverse cholesterol transport (RCT), are covered in fine detail in
Chapters 7,
8, and
9, respectively. Only a brief overview is provided here to provide a background to discuss the observation studies, the randomized controlled clinical trials, and the disorders of lipid and lipoprotein metabolism and their relevance to the diagnosis and treatment of dyslipidemia germane to pediatrics.
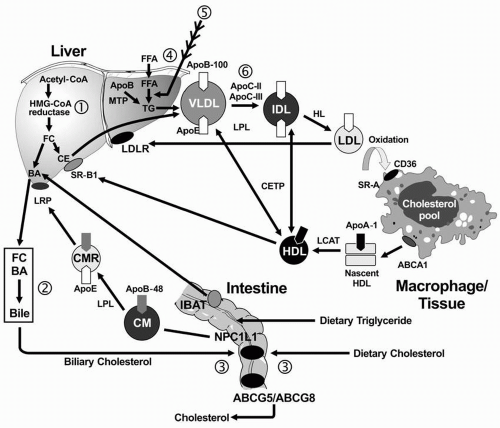
Exogenous Lipid Transport. In the small intestine, lipids are emulsified by bile salts and hydrolyzed by pancreatic lipases. The bile acids are then reabsorbed by the intestinal bile acid transporter (IBAT) for return to the liver through the entero-hepatic pathway (
Fig. 12.1). TG are broken down into FFA acids and monoglycerides; cholesteryl esters (CE) are hydrolyzed into FFA and unesterified cholesterol. These components are then absorbed by the intestinal cells. The absorption of cholesterol occurs in the jejunum, through the high-affinity uptake of dietary and biliary cholesterol by the Niemann-Pick C1 Like 1 (NPC1L1) protein (
Fig. 12.1). Normally, about half the dietary and biliary cholesterol is absorbed daily. Excessive cholesterol absorption is prevented by the ATP binding casette (ABC) transporters, ABCG5/ABCG8, which act together to pump excess cholesterol and plant sterols from the intestine back into the lumen for excretion into the stool (see
Fig. 1.4) In intestinal cells, monoglyceride is re-esterified into TG and cholesterol is esterified by acyl cholesterol acyltransferase (ACAT), and both lipids and apoB-48 and other apolipoproteins are packaged into chylomicrons.
After absorption of dietary fat, the CE and TG are packaged with apoB-48 to form chylomicrons, which are then secreted from intestinal cells. The enzyme lipoprotein lipase (LPL) on the surface of endothelial cells (with apoC-II required as a cofactor) hydrolyzes the TG into free fatty acids (FFA) and monoglycerol. The released FFA are primarily taken up by adipose tissue (for storage) and muscle cells (for energy). The components that remain constitute the “chylomicron remnants,” which are removed from the blood in the liver by interaction of apoE with the chylomicron remnant receptor, also called the low density lipoprotein receptor-related protein (LRP).
Endogenous Lipid Transport. TG-rich VLDL is secreted by the liver into plasma, a process requiring apoB-100 (
Fig. 12.1). The VLDL-TG are subsequently hydrolyzed by LPL and apoC-II
into FFA and monoglycerols, resulting in the formation of VLDL remnants and subsequently IDL. ApoE is the ligand for the hepatic uptake of some of the IDL particles by the LDL receptor (LDLR), while the remaining IDL are hydrolyzed by LPL and hepatic lipase (HL), producing the final product of VLDL catabolism, namely LDL. LDL are normally removed from plasma following the binding of apoB-100 to the hepatic LDLR (
1).
Reverse Cholesterol Transport. RCT refers to the pathway by which cholesterol is transported away from peripheral cells, such as macrophages and foam cells in the walls of blood vessels to the liver for uptake and excretion into bile. Free cholesterol is removed from peripheral cells by the interaction of apoA-I on the nascent HDL particle with the ATP-binding casette (ABC) transporter, ABCA1. The cholesterol in the nascent HDL is esterified by lecithin-cholesterol acyltransferase (LCAT), with apoA-I as a cofactor, producing a more mature and larger HDL particle with cholesteryl ester in its core. The mature HDL can deliver the cholesteryl ester directly to the liver through the interaction of apoA-I with the HDL receptor (also called scavenger receptor, class B, type I [SR-BI];
Fig. 12.1). The cholesterol derived from this process may be excreted into bile either as cholesterol or by conversion of cholesterol into bile acids. Less than half of the cholesterol from peripheral cells is delivered to the liver through this pathway. The remaining CE is transferred from HDL to the apoB-containing lipoproteins in exchange for TG by the cholesteryl ester transfer protein (CETP).
Relationship between Atherosclerosis and CVD Risk Factors
Pathologic Studies. Several longitudinal pathologic studies from the general population found that early atherosclerotic lesions of fatty streaks and fibrous plaques in children, adolescents, and young adults, who died from accidental deaths, are significantly related to higher antecedent levels of total cholesterol (TC) and LDL cholesterol (LDL-C), lower levels of HDL cholesterol (HDL-C), and other CVD risk factors, such as obesity, higher blood pressure, and cigarette smoking (
1,
2). These effects of risk factors on coronary lesion severity are multiplicative rather than additive.
Prospective Epidemiologic Studies. Five major prospective population studies—two from Muscatine and Bogalusa, and the Coronary Artery Risk Development in Young Adults (CARDIA), the Special Turku Coronary Risk Factor Intervention Project (STRIP), and the Cardiovascular Risk in Young Finns Study—showed that CVD risk factors in children and adolescents, particularly LDL-C and obesity, predicted clinical manifestations of atherosclerosis in young adults, as judged by coronary artery calcium, carotid intima-media thickness (IMT), or brachial flow-mediated dilatation (FMD) (
1,
2,
3,
4). In contrast, HDL-C levels in childhood and early adulthood have been related to decreased IMT and increased FMD (
3). There are little data on prediction of CVD events; however, medical students at Johns Hopkins who had a TC level >207 mg/dL had five times the risk of developing CVD 40 years later than those who had a TC level <172 mg/dL (
1).
Human Genetic Studies. Studies have also been performed in
high-risk youth selected by virtue of CVD in one parent or because they have inherited a known metabolic disorder of lipoprotein metabolism that produces premature CVD. Half of the young progeny of men with premature CVD before 50 years of age had one of seven dyslipidemic profiles: elevated LDL-C alone (type IIa) or combined with high TG (type IIb); elevated TG alone (type IV); low HDL-C alone (hypoalpha); and type IIa, type IIb, or type IV also accompanied by low HDL-C (
1). Elevated levels of apoB, in the presence of normal LDL-C (hyperapobetalipoproteinemia or hyperapoB), were prevalent in young offspring of adults with premature CVD and hyperapoB (
1). The levels of apoB and apoA-I, the major apolipoproteins of LDL and HDL, respectively, and the ratio of apoB to apoA-I in young offspring from Bogalusa, were stronger predictors of premature coronary artery disease (CAD) in their parents than LDL-C and HDL-C levels (
1). Related findings were recently reported from the Cardiovascular Risk in Young Finns Study, where childhood levels of serum apoB and apoA-I predicted carotid IMT and brachial FMD in adulthood better than LDL-C and HDL-C (
3).
Inherited Disorders of Lipoprotein Metabolism. Examples of inherited lipoprotein disorders that often present in youth at high risk of future CVD include familial hypercholesterolemia (FH), due to a defect in the LDLR, and familial combined hyperlipidemia (FCHL), and its metabolic cousin, hyperapoB, the prototypes for hepatic overproduction of VLDL, which are often accompanied by insulin resistance and the dyslipidemic triad of hypertriglyceridemia, increased small, dense LDL particles (LDL-P), and low HDL-C (see also the following text). Recently, the presence of a type IIb phenotype (elevated LDL-C and TG) in childhood predicted increased carotid IMT, elasticity, and FMD in early adulthood (
4).