Disorders of Swallowing: Introduction
Demographic changes related to aging necessitate that clinicians have the resources to address eating and swallowing difficulties present in older adults. The capacity to effectively and safely eat or swallow is one of the most basic human needs and also can be a great pleasure. Therefore the loss of this capacity can have far-reaching implications. Many would argue that swallowing is one of the cardinal behaviors needed to sustain life. The process of swallowing requires orchestration of a complex series of psychological, sensory, and motor behaviors that are both voluntary and involuntary. Dysphagia refers to difficulty swallowing that may include oropharyngeal or esophageal problems. More specifically, there may be difficulty in oral preparation for swallowing and/or moving material from the mouth to the esophagus and from the esophagus to the stomach.
Although age-related changes place older adults at risk for dysphagia, an older adult’s swallow is not inherently impaired. Presbyphagia refers to characteristic changes in the mechanism of swallowing of otherwise healthy older adults. Clinicians need to be able to distinguish among dysphagia, presbyphagia, and other related diagnoses such as globus hystericus to avoid overdiagnosis and overtreatment of dysphagia. Older adults can be more vulnerable and, with additional stressors such as acute illness and certain medications, they can cross over from having a healthy older swallow (presbyphagia) to experiencing dysphagia. This chapter reviews the normal swallowing process and presbyphagia, as a healthy aging evolution, dysphagia outcomes, multidisciplinary approaches to diagnosing and managing dysphagia, and newly recognized rehabilitation strategies for dysphagia care.
Impact of Dysphagia
Dysphagia prevalence depends on the specific population sampled, with community-dwelling and more independent individuals having rates near 15%. Upward of 40% of people living in institutional settings such as assisted-living and nursing homes are dysphagic. With the projected growth in the number of individuals living in nursing homes, there is a compelling need to address dysphagia not only in ambulatory and acute care settings but also in long-term care settings.
The consequences of dysphagia vary from social isolation because of the embarrassment associated with choking or coughing at mealtime to physical discomfort (e.g., food sticking in the chest) to potentially life-threatening conditions. The more ominous sequelae include dehydration, malnutrition, and both overt and silent aspiration precipitating pulmonary complications. For the purposes of this chapter, aspiration is defined as the entry of material into the airway below the level of the true vocal folds. Silent aspiration refers to the circumstance in which a bolus comprising saliva, food, liquid, medication, or any foreign material enters the airway below the vocal folds without triggering overt symptoms such as coughing or throat clearing. Both overt and silent aspiration may lead to pneumonitis, pneumonia, exacerbation of chronic lung disease, or even asphyxiation and death. To gain a better understanding of the effect, these consequences have on older adults and the impact of dysphagia interventions, research in this area has aimed to develop more meaningful outcome measures. Assessments focused on pathophysiology, function, and health services are now being conducted to create more evidence-based practices in dysphagia care.
Insofar as dysphagia is a biomechanical disorder of bolus flow, signs of flow abnormality, using videofluoroscopy, have been well detailed. These include (1) the duration, direction, and completeness of bolus flow; (2) the duration and extent (range) of anatomic structural movements; and (3) the relationship between bolus flow and structural movements as well as between physiologic and anatomic parameters such as pressure generation and muscle/fat structure. More recently, the bulbar innervated swallowing mechanisms are being studied and are providing targets specific for novel treatment paradigms aimed at improving swallowing function. Other clinical outcomes of dysphagia have become important endpoints in assessing interventions that aim to make it possible for patients to eat and drink adequately and safely. These include measures of hydration, nutrition, aspiration episodes, and emotional sequelae. Other measures have been developed that target what and how dysphagic adults eat and drink. Most of these scales are directly applicable to older adults (Table 41-1). Additionally, pneumonitis, overt aspiration pneumonia, and additional forms of evidence of pulmonary damage are monitored. Nonetheless, it has been difficult to attribute mortality directly to dysphagia because it is often a secondary rather than a primary diagnosis.
TOOL | COMPONENTS |
|---|---|
American Speech Language Hearing Association Scale (1996) |
|
Rehabilitation Institute of Chicago Clinical Evaluation of Dysphagia (Cherney et al, 1986) |
|
Amyotrophic Lateral Sclerosis Severity Scale (Hillel et al, 1989) |
|
Dysphagia Disability Index (Silbergleit et al, in progress) |
|
Dysphagia Severity Rating Scale (Waxman et al, 1990) |
|
Functional Outcome Swallowing Scale (Salassa, 1997) |
|
Functional Outcome Assessment of Swallowing (Wisconsin Speech and Hearing Association, 1996) |
|
Dysphagia profoundly influences quality of life (QOL). Patients with swallowing difficulties, especially those who relinquish oral eating, manifest significant changes in psychosocial status, functional status, and emotional well-being. Eating and drinking are social events that relate to friendship, acceptance, entertainment, and communication. As such, major adjustments in the process of feeding and eating can lead to distressing responses such as shame, anxiety, depression, and isolation. Only recently have practical dysphagia-specific, comprehensive, QOL measures been developed. By monitoring functional outcomes in clinical practice, physicians and other health care providers may be able to better assess and adjust their treatment of dysphagia.
Swallowing Process
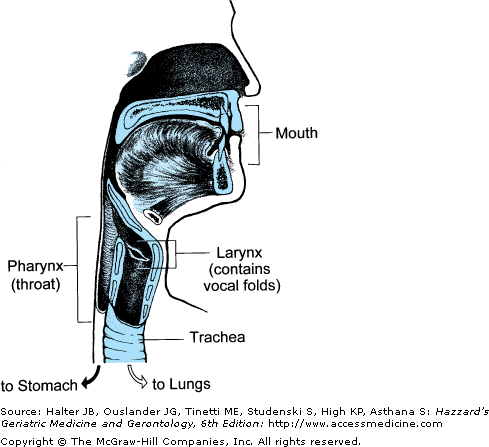
Swallowing is an orchestrated activity that balances the competing behaviors of ingestion, speaking, and breathing. Approximately 30 oral and pharyngeal muscle pairs and multiple nerves must perform precisely on cue so that the upper aerodigestive tract is reconfigured from a mechanism that channels air for breathing and speaking (Figure 41-1) to a food-propelling mechanism that accomplishes ingestion (Figure 41-2). The four morphologic regions serving these purposes are the oral cavity, pharynx, larynx, and esophagus. Of these, the first three collectively are termed the upper aerodigestive tract because they also serve the airway-dependent functions of respiration and speech production. In humans, with our upright posture, it is the adjacent position of the anatomy for breathing to the anatomy for food passage that facilitates gravitational influences on food to flow into an unprotected airway. Such anatomy and physiology require precision to satisfy the delicate balance between swallowing physiology and breathing—each a life-sustaining function that must occur during cessation of its counterpart. Thus, a basic understanding of the relationship between the anatomic components and the functional interaction of this mechanism is essential to an understanding of normal swallowing and the effects of age and age-related diseases on it.
Figure 41-1.
Aerodigestive tract channeling air for breathing from the nose and mouth through the open larynx into the lungs and back up and out. For speaking, air is channeled similarly, but the vocal folds vibrate to produce voice. (Adapted with permission from Weihofen D et al. Shape. The Easy to Swallow, Easy to Chew Cookbook. New York, New York: John Wiley and Sons; 2002.)
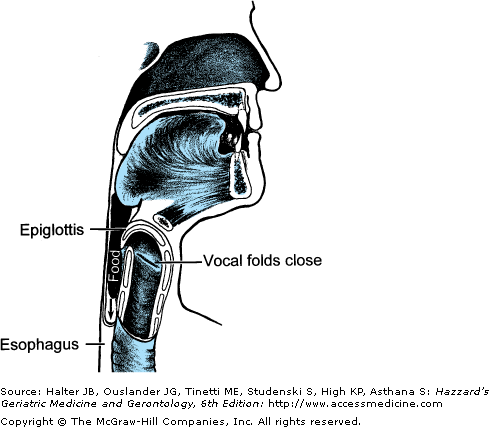
Figure 41-2.
Aerodigestive tract reconfigured from an air channel to a food-propelling mechanism during swallowing. The tongue propels food into the throat; the epiglottis covers the larynx, which is the airway entrance; and the vocal folds close to protect the trachea and lungs from foreign material. (Adapted with permission from Weihofen D et al. The Easy to Swallow, Easy to Chew Cookbook. New York, New York: John Wiley and Sons; 2002.)
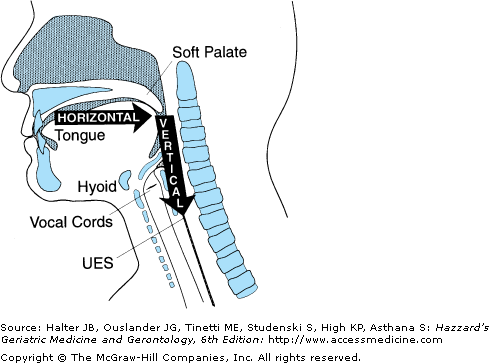
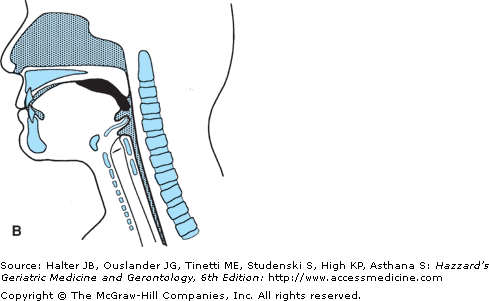
Swallowing is an integrated neuromuscular process consisting of a combination of volitional and relatively automatic movements. Although normal swallowing is usually conceptualized as a continuous sequence of events, the process of deglutition has been conveniently described as occurring in two, three, or four phases or stages. Moreover, the system engaged in swallowing can be divided into two basic structural subsystems, horizontal and vertical (Figure 41-3), that mirror the direction of bolus flow and the potential for gravitational influence on it.
Figure 41-3.
The oropharyngeal swallowing mechanism can be divided into two basic structural subsystems, horizontal and vertical, that mirror the direction of bolus flow. UES, upper esophageal sphincter. (Adapted with permission from Robbins JA. Normal swallowing and aging. Semin Neurol. 1996; 16(4):309.)
The oral cavity components comprise the horizontal subsystem. As such, this subsystem is involved in the initial, largely volitional, processing and transport phases of swallowing—the swallow preparatory phase and the oral transport phase. The swallow preparatory phase is characterized by food acceptance, containment, and manipulation. The lips and the buccal musculature act in complex patterns, varying the size and shape of the oral opening to allow acceptance and/or containment of food within the oral cavity. The process of chemically changing the material requires that numerous labial and lingual glands secrete into the oral cavity an enzyme-rich fluid that maintains and lubricates the mucosa and is directly incorporated into the food. This textural manipulation of food and the mechanical formation of a bolus are accomplished largely by the tongue. The tongue positions the bolus between the teeth and moves in a complex three-dimensional chewing pattern if the bolus requires mastication. The moistening of the food in the oral cavity is essential for normal bolus transit and/or flow and clearance, particularly because gravity provides no assistance until the vertical phases of swallowing.
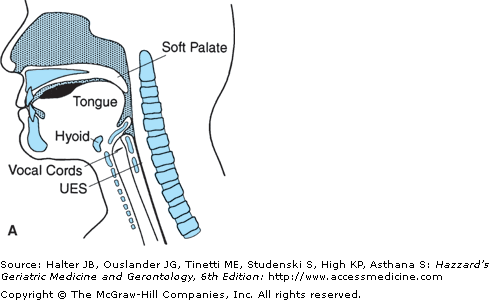
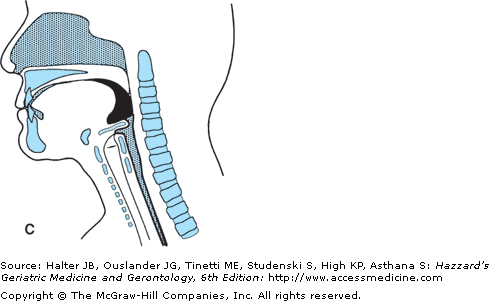
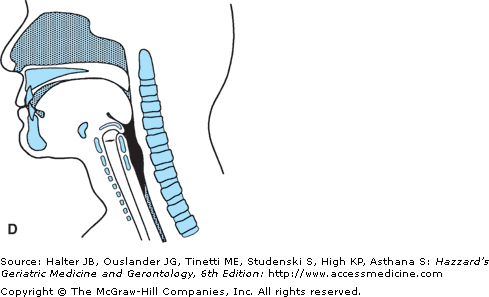
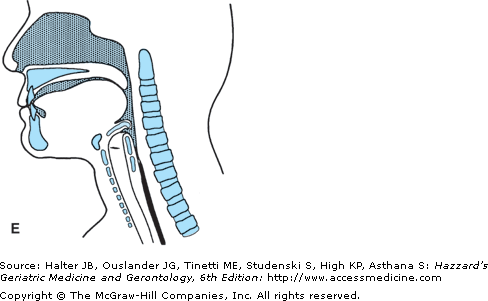
The oral transport phase comprises movement of the cohesive bolus (masticated if necessary) posteriorly (and horizontally when the subject is in a normal upright seated posture) to the inlet of the superior aspect of the vertical subsystem, the pharynx (Figure 41-4). The intrinsic tongue muscles change the shape of the tongue, forming grooves along its body and anterior and lateral seals to facilitate containment. The extrinsic tongue muscles change the position of the tongue within the oral cavity and assist in changing its shape (Figure 41-4A), and then progressively arching the tongue posteriorly to transport the bolus to the vertical subsystem (Figures 41-4B and C).
Figure 41-4.
Lateral view of bolus propulsion during swallowing. (A) Voluntary initiation of the swallow by tongue “loading.” (B) Bolus propulsion by tongue dorsum and UES opening anticipating bolus arrival. (C) Bolus entry into pharynx associated with epiglottal downward tilt, hyolaryngeal excursion, and UES opening. (D) Linguapharyngeal contact facilitating bolus passage through the pharynx and (E) UES closing and completion of oropharyngeal swallowing; then the entire bolus is in the esophagus. (Adapted from Robbins JA: Normal swallowing and aging. Semin Neurol. 16(4):309–317, Dec., 1996.)
The pharyngeal and laryngeal components, in conjunction with the tongue dorsum, comprise the superior aspect of the vertical subsystem, where gravity begins to assist in the transport of the bolus. The anatomic juxtaposition of the entrance to the airway (laryngeal vestibule) and the pharyngeal aspect of the upper digestive tract demand biomechanical precision to ensure simultaneous airway protection and bolus transfer or propulsion through the pharynx. To this end, the pharyngeal transport phase is characterized by a sequence of rapid, highly coordinated neuromuscular events that cause pressure changes critical to bolus transport or transit in a safe, timely, efficient manner.
Linguapalatal contact sequentially moves the bolus against the posterior pharyngeal wall, contributing to the positive pressures imparted to the bolus and propelling it downward. Simultaneously, the pharyngeal constrictors begin to contract in a descending sequence, first elevating and widening the entire pharynx to engulf the bolus (Figure 41-4D and E), and clean up residue after swallowing is completed. A descending peristaltic wave then cleanses the pharynx of residue. Tight closure of the velopharynx during the pharyngeal transport phase provides a seal at the superior aspect of the vertical system, preventing nasal leakage of the bolus and contributing to the generation of high positive pressures, which are applied to the bolus.
Several mechanisms ensure the redundancy by which airway protection is accomplished. Three levels of sphincteric closure include (1) the aryepiglottic folds, (2) the false vocal folds, and (3) the true vocal folds, with closure of the true vocal folds (the lowest of the three sphincters) providing “the last line of defense” to prevent aspiration of invasive material. The hyolaryngeal complex is lifted upward and forward by the combined contraction of the suprahyoid muscles, thyrohyoid muscles, and pharyngeal elevators. This hyolaryngeal elevation and anterior movement, coupled with retraction of the tongue base, covers the laryngeal vestibule and diverts the bolus laterally around the airway. Timely relaxation and opening of the upper esophageal sphincter (UES) permits continuous vertical passage of the bolus into the esophagus. The UES functions as a mechanical valve. For it to open normally, four criteria must be met: (1) relaxation of muscle tone, (2) compliant tissue, (3) traction force provided by sufficient hyolaryngeal excursion, and (4) pulsion force imparted by the bolus. In normal swallowing, UES relaxation and opening occur prior to bolus arrival at the hypopharynx.
Historically, swallowing was largely viewed as a sequence of pharyngeal and esophageal events and was defined as reflexive. The findings from quantitative temporospatial studies on normal swallowing conducted in the last two decades, and current knowledge of the underlying neural substrates, have provided new insights into oropharyngeal swallowing, showing that it is a patterned response rather than a traditional reflex.
Sensorimotor control of swallowing requires the coordinated activity to be distributed across both the cranial and spinal nerve systems, including the peripheral nerves, their central nuclei, and their neural centers. More specifically, the neural control of swallowing involves five major components: (1) afferent sensory fibers contained in cranial nerves, (2) cerebral and midbrain fibers that synapse with the brainstem swallowing centers, (3) paired swallowing centers in the brainstem, (4) efferent motor fibers contained in cranial nerves and the ansa cervicalis, and (5) muscle and end organs. Indeed, this neural network spans all levels of the neuraxis from the cerebrum superiorly to the brainstem and spinal nerves inferiorly and muscles and end organs at the periphery. This relatively diffuse network is designed to integrate and sequence both the volitional and automatic actions of swallowing.
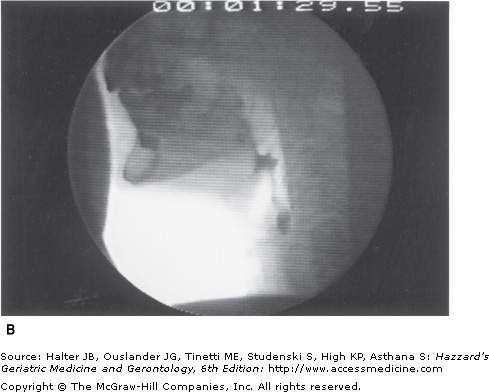
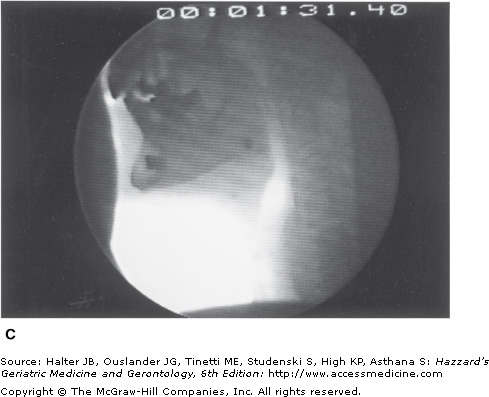
Healthy persons depend on a highly automated neuromuscular sensorimotor process that intricately coordinates the activities of chewing, swallowing, and airway protection. To accomplish a normal swallow, which occurs in 2 seconds or less, the muscles of chewing interact with 26 pairs of striated pharyngeal and laryngeal muscles. The muscles involved in chewing include the masseters, temporalis, and pterygoids (innervated by cranial nerve V); the lip and buccal musculature, the orbicularis oris, and the buccinator (innervated by cranial nerve VII); and the intrinsic and extrinsic lingual muscles (innervated by cranial nerve XII). Optimal structural integrity and precise neural mediation result in continuous, rapid bolus flow from the mouth to the esophagus (Figure 41-5A, B and C) that accommodates variations in bolus size, texture, and temperature and the individual’s intent to swallow, chew, or just hold the bolus in the mouth.
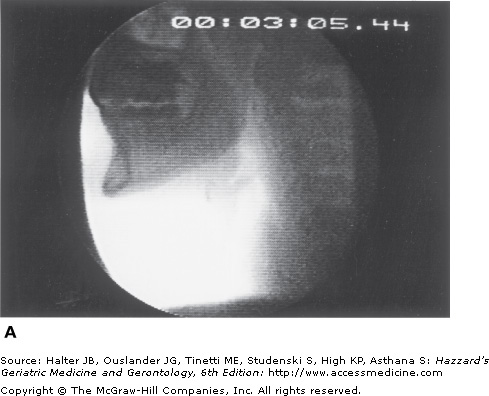
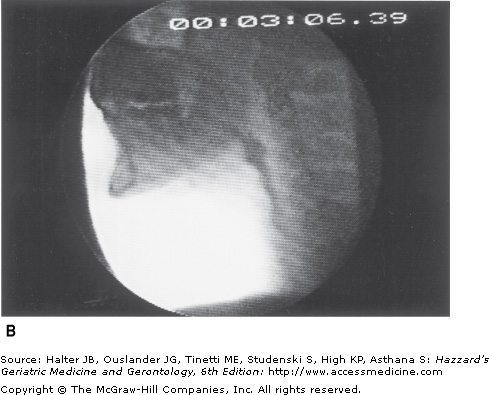
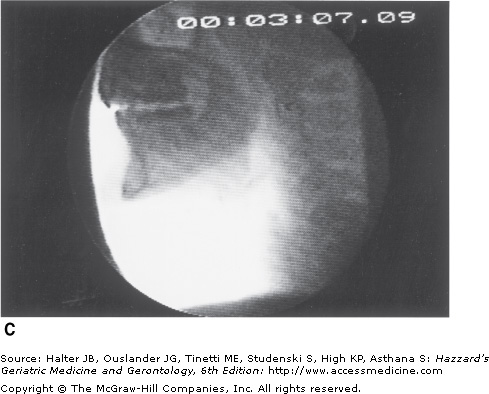
Figure 41-5.
Healthy young swallowing documented with videofluoroscopy. (A) Bolus in oral cavity, ready to be swallowed. (B) Bolus appears as a “column” of material swiftly moving through the pharynx. (C) Oropharynx cleared of material when the swallow is completed. (Adapted with permission from Robbins JA. Normal swallowing and aging. Semin Neurol. 1996; 16(4):309.)
Research on swallowing neurophysiology over the past decade has begun to reveal complex underlying neural substrates that may provide an understanding of how willful control during the early stage of swallowing allows access to the patterned response. Behavioral and sensory interventions attempting to modify a response certainly have more treatment potential than if they are aimed at a reflex. Accessing mechanisms of neural plasticity, defined as the ability of the brain to change, is becoming a focus to elicit neuroprotection relative to aging effects or recovery from dysphagia following injury such as stroke.
Senescent Swallowing
Traditional thinking suggests that the causes of dysphagia are always disease-related, with direct or indirect damage to effector end-organ systems of swallowing. Research during the past decade has indicated that swallowing changes occur even with healthy aging. This work, with a focus primarily on the anatomy and physiology of the oropharyngeal swallowing mechanism, describes a progression of change that may put the older population at increased risk for dysphagia. This research is particularly relevant when an older healthy adult, whose functional reserve is naturally diminished, is faced with increased stressors such as central nervous system (CNS)-altering medications, mechanical perturbations (e.g., a nasogastric [NG] tube or a tracheostomy), or chronic medical conditions (e.g., frailty) that might not elicit dysphagia in a less vulnerable individual. Translation of this work into clinical practice will provide safeguards against the overdiagnosis and overtreatment of dysphagia in the elderly population.
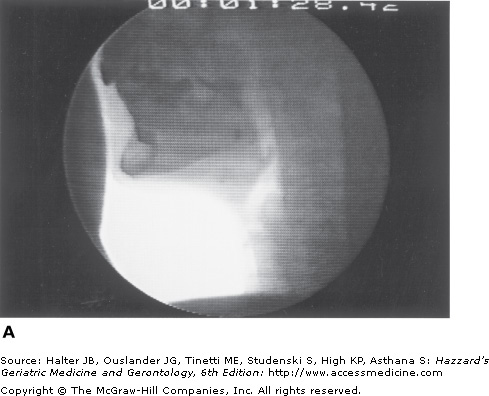
A major characteristic of older healthy swallowing is that it occurs more slowly. In people older than 65 years, the initiation of laryngeal and pharyngeal events, including laryngeal vestibule closure, maximal hyolaryngeal excursion, and upper UES opening, takes significantly longer than in adults younger than 45 years. Although the specific neural underpinning has not been confirmed, oral events may become uncoupled from the pharyngeal response. Thus, in older healthy adults, the bolus may remain adjacent to an open airway by pooling or pocketing in the pharyngeal recesses longer than in younger adults (Figure 41-6).
Figure 41-6.
Healthy old swallowing documented with videofluoroscopy. (A) Bolus in mouth ready for swallowing. (B) Bolus pooled in vallecula and pyriform sinus during delayed onset of pharyngeal response. (C) Bolus cleared of material when the swallow is completed. (Adapted with permission from Robbins JA. Normal swallowing and aging. Semin Neurol. 1996; 16(4):309.)
Aspiration and airway penetration are believed to be the most significant adverse clinical outcomes of misdirected bolus flow, as reflected in the high rates of pneumonia with increasing age and disease. However, airway invasion in healthy adults is commonly believed to be largely absent. The use of an eight-point scale (Table 41-2) that discretely quantifies the occurrence, the depth, and the person’s reaction to airway penetration or aspiration found no significant difference between young (21- to 32-year-olds) and old (63- to 84-year-olds) age groups in scale scores on three repeat swallows. However, when a greater number of swallows were performed, aspiration did occur in the older group, indicating fatigue or change in endurance as a possible factor that may relate in a very practical sense to eating meals.
| CATEGORY | SCORE | DESCRIPTION |
|---|---|---|
No penetration or aspiration | 1 | Contrast does not enter the airway |
P E N | 2 | Contrast enters the airway, remains above vocal folds, no residue |
E T R | 3 | Contrast remains above vocal folds, visible residue remains |
A T | 4 | Contrast contacts vocal folds, no residue |
I O N | 5 | Contrast contacts vocal folds, visible residue remains |
A S P | 6 | Contrast passes glottis, no subglottic residue visible |
I R A | 7 | Contrast passes glottis, visible subglottic residue despite patient’s response |
T I O N | 8 | Contrast passes glottis, visible subglottic residue, absent patient response |
Using simultaneous videofluoroscopy and manometry (manofluoroscopy) and a tube through the nasopharynx similar in size to a “garden variety” NG tube, a significant interaction was found for tube by age. That is, liquid penetrated the airway significantly more frequently when the tube was in place only in the oldest group of men and women (older than 70 years). Thus, it appears that under demanding conditions (e.g., endurance = demanding, or NG tube placement), older individuals are less able to compensate and are more at risk for aspiration. Findings such as these characterize presbyphagia or “old swallowing” which is not abnormal swallowing in the elderly population.
Age-related changes in the generation of lingual pressure also define presbyphagia. Healthy older individuals have reduced isometric tongue pressures compared with younger individuals. In contrast, the generation of maximal lingual pressure during swallowing (which requires submaximal pressures) remains “young” in magnitude but slows in the time necessary to achieve those “young” swallowing pressures. The relationship between maximum isometric pressure and peak swallowing pressures can be considered an indication of the functional reserve available for swallowing. As people get older, slower swallowing may allow time to recruit the necessary number of motor units required for pressures critical for adequate bolus propulsion through the oropharynx. However, fluids of low viscosity (e.g., water, tea), by their composition, are likely to move more quickly than the physiology to handle them safely and thus put older people at increased risk for aspiration.
Neuroimaging studies using cranial magnetic resonance imaging (MRI) in normal adults have shown a relationship between slower swallowing and an increase in the number and severity of periventricular white-matter hyperintensities (PVHs) in the brain, supporting the concept that voluntary control of swallowing is mediated by corticobulbar pathways traveling within the periventricular white matter. The occurrence and degree of PVHs increase with age and may explain, at least in part, the relatively asymptomatic decline in oropharyngeal motor performance observed in older people. Cerebral atrophy, a common finding in asymptomatic older individuals, may be another contributing factor to presbyphagia.
Changes in the periphery also occur with age and may be a function of changes in various sensory mechanisms or caused by muscle atrophy. Similar to the age-related loss of limb skeletal muscle, are the changes with age in fiber density, muscle tension, muscle strength, and muscle contraction in facial, masticatory, and lingual musculature. Rather than reflecting CNS deterioration, slowed swallowing that remains coordinated and effective, as found in most healthy old people, may represent a compensatory strategy for achieving pressure-generation values that may be critical to successful bolus propulsion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree