Figure 101.1 Spectrum of opportunistic infections in untreated HIV infection and the CD4-T-cell-count ranges in which they manifest. CMV = cytomegalovirus; CNS = central nervous system; PCP = Pneumocystis jirovecii pneumonia.
Mucocutaneous infections
Thrush and angular cheilitis are the major manifestations of oropharyngeal candidal infection in HIV-infected patients. Thrush is particularly common and often heralds the presence of other more serious OIs (Figure 101.1). It typically presents as asymptomatic, white, loosely adherent deposits on the dorsum of the tongue but frequently extends posteriorly to involve the palate and oropharynx. Severe cases are associated with oral discomfort, dysgeusia, and nausea. Angular cheilitis is rarer and presents as painful, sometimes bleeding sores at the corners of the mouth. Treatment of both conditions consists of topical or oral antifungal therapy (Table 101.1).
| Condition/pathogen | First-line treatment | Notes |
|---|---|---|
| Thrush or candidal angular cheilitis | Clotrimazole troches, 10 mg PO 5 × daily; nystatin solution, swish and swallow QID; or fluconazole, 100–200 mg PO qd for 7–14 days | In the event of azole resistance: amphotericin B suspension, 100 mg/mL PO QID; amphotericin B deoxycholate, 0.3–0.7 mg/kg IV qd; liposomal or lipid complex amphotericin, 3–5 mg/kg; voriconazole, 200 mg PO qd; or caspofungin 50 mg IV qd for 7–14 days |
| Seborrhea dermatitis | Face: imidazole cream (ketoconazole 2% or clotrimazole 1%) plus hydrocortisone 1.0%–2.5% or desonide 0.05% cream BID Scalp/body: antidandruff shampoo (e.g., Selsun Blue or Head and Shoulders) plus triamcinolone 0.1% cream (body) or solution (scalp) | Severe cases may require addition of oral ketoconazole, 200–400 mg qd for 2–4 weeks |
| Varicella-zoster virus | Acyclovir, 800 mg PO 5 × daily; famciclovir, 500 mg PO TID; or valacyclovir, 1000 mg PO TID for 7–10 days | (1) Trigeminal or disseminated cutaneous zoster; attendant meningoencephalitis; or evidence of visceral involvement (i.e., elevated transaminases and/or pancreatic enzymes) all merit IV acyclovir, 10 mg/kg q8h, until clinical resolution (minimum 14–21 days). (2) In the event of acyclovir resistance: foscarnet, 40–60 mg/kg IV q8h, or cidofovir plus IV hydration and oral probenecid to mitigate renal toxicity |
| Herpes simplex virus | Acyclovir, 400 mg PO TID; famciclovir, 500 mg PO BID; or valacyclovir, 1000 mg PO BID for 7–14 days | In the event of acyclovir resistance: foscarnet, 40–60 mg/kg IV q8h, or cidofovir plus IV hydration and oral probenecid to mitigate renal toxicity |
| Molluscum contagiosum | Laser, cryotherapy, and curettage plus HAART | |
| Oral hairy leukoplakia (OHL) | HAART | (1) OHL is pathognomonic of underlying HIV infection. (2) Rarely warrants specific therapy; but oral acyclovir 800 mg PO 5 × daily and podophyllin have been used with variable success in severe cases |
Abbreviations: PO = orally; QID = four times per day; qd = every day; BID = twice per day; TID = three times per day; HAART = highly active antiretroviral treatment; IV = intravenously.
Seborrhea dermatitis is a greasy, flaky, and faintly erythematous rash with a predilection for the face. Areas rich in sebaceous glands – the hairline, eyebrows, nose, and nasolabial folds – are primary targets. The role of Malazesia furfur infection in pathogenesis remains uncertain. Topical antifungal and corticosteroid agents are used to treat this condition (Table 101.1).
Recurrent or multidermatomal cutaneous herpes zoster infections are sometimes the only outward indication of waning cell-mediated immunity in HIV-infected adults. Lancinating pain and paresthesias in the soon-to-be involved dermatome are followed days later by the sudden outcropping of a vesicular rash on an erythematous base. Use of oral acyclovir or related agents (valacyclovir or famciclovir) within 72 hours improves symptoms, speeds up crusting, and may reduce the risk of postherpetic neuralgia (Table 101.1).
Mucocutaneous herpes simplex virus (HSV) infections are more frequent at CD4 counts ≤500 cells/mm3. These primarily manifest as painful vesicles in the genital and perirectal areas. Oral acyclovir, valacyclovir, and famciclovir are all effective in reducing the severity and duration of symptoms. They are also effective prophylactically (Table 101.1).
Molluscum contagiosum is a poxvirus that causes characteristically firm, umbilicated, and sometimes pedunculated skin lesions (Figure 101.2). Intertriginous areas of the body, namely the axillae, perineum, antecubital, and popliteal fossae, are disproportionately involved. Transmission requires intimate physical contact with an infected person. No specific chemotherapy exists; however, effective antiretroviral therapy can hasten lesion regression. Alternatively, lesions can be removed by cryotherapy, curettage, or laser cautery (Table 101.1).
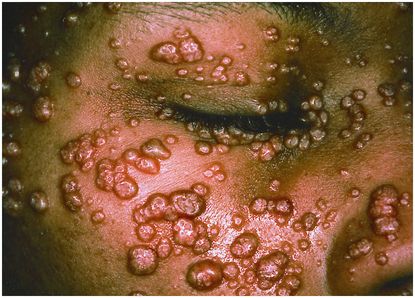
Figure 101.2 Molluscum contagiosum. Numerous flesh-colored umbilicated papules appeared on the face of a 33-year-old man with a three-year history of the acquired immunodeficiency syndrome and a CD4 cell count of 60 cells/µL. Prominent lesions along the margin of the eyelids prevented him from closing his eyes completely. Reproduced with permission from Stephanie Cotell. Cotell SL, Roholt NS. N Engl J Med. 1998 Mar 26;338(13):888. Northwestern University, Chicago, IL 60611, USA.
Oral hairy leukoplakia is virtually pathognomonic of underlying HIV infection. These characteristically painless, white, tightly adherent vertical ridges on the lateral aspect of the tongue are outward manifestations of Epstein–Barr virus infection of lingual squamous epithelium. Robust immune recovery through HAART is the most effective treatment for this condition (Table 101.1).
Pulmonary infections
Three elements govern the initial evaluation and management of lower respiratory infections in HIV-positive patients: (1) prompt administration of empiric antibiotics against community-acquired or healthcare-associated pneumonia pathogens; (2) immediate respiratory isolation for individuals presenting with either symptoms or chest imaging findings concerning for pulmonary tuberculosis; and (3) early administration of empiric treatments for Pneumocystis jirovecii (carinii) pneumonia (PCP) in individuals presenting with evidence of advanced HIV disease plus either acute respiratory failure and/or radiographic findings suspicious for PCP (discussed below).
Computed tomography (CT) scans, given their increased sensitivity and superior image resolution over chest roentgenograms (CXR), are frequently necessary in evaluating patients with AIDS. This is particularly true in cases where the CXR shows either nodular/cavitary infiltrates or suggests the presence of mediastinal or hilar lymphadenopathy. The vast array of diagnostic possibilities in such cases (including noninfectious causes such as metastases from cancer) makes devising empiric therapeutic regimens unwieldy (Table 101.2). Thus, CT imaging is often helpful in refining and paring down empiric chemotherapeutic choices pending definitive results from sputa, bronchoscopy or lung biopsy.
| Chest x-ray or computed tomography findings | Common pathogens |
|---|---|
| (1) Focal/lobar asymmetric consolidation without intrathoracic lymphadenopathy | Streptococcus pneumoniae, Moraxella catarrhalis, Haemophilus influenzae; Mycobacterium tuberculosis in individuals with primary infection or CD4 counts >350 cells/mm3 |
| (2) Diffuse interstitial or alveolar infiltrates without intrathoracic lymphadenopathy | Pneumocystis jirovecii, respiratory viruses (e.g., influenza virus and respiratory syncytial virus), Mycoplasma pneumoniae |
| (3) Reticulonodular and/or cavitating nodular infiltrates without intrathoracic lymphadenopathy (acute presentations) | Mycoplasma pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa |
| Reticulonodular and/or cavitating nodular infiltrates plus intrathoracic lymphadenopathy (indolent presentations) | Mycobacterium tuberculosis, Norcardia asteroides, Aspergillus fumigatus, Rhodococcus equi |
Pneumocystis jirovecii pneumonia
Low-grade fever, dry cough, and progressive dyspnea are the cardinal symptoms of Pneumocystis jirovecii pneumonia (aka PCP). PCP is the most frequently identified cause of lower respiratory infection in HIV-infected patients. Risk factors include CD4 counts ≤200 cells/mm3, CD4 percentage ≤14, prior PCP, and thrush. Radiographically, PCP usually presents as symmetric, perihilar interstitial or alveolar infiltrates (Figure 101.3). However, excluding PCP purely on the basis of CXR findings can be hazardous. For example, follow-up CTs sometimes uncover classic PCP findings where prior CXRs had either been clear or suggestive of a bacterial process. Secondly, a number of “atypical” radiographic findings – including cystic, apical, nodular, and cavitary infiltrates – have also been described in cytologically proven cases of PCP.
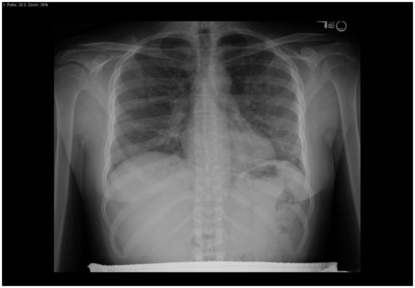
Figure 101.3 Pneumocystis jirovecii pneumonia (PCP). Bilateral interstitial infiltrates on chest x-ray in a 39-year-old woman with AIDS and CD4 count of 33 cells/mm3.
Intravenous trimethoprim–sulfamethoxazole (TMP–SMX) is the first-line agent for treating severe PCP (Table 101.3). In patients with sulfa allergies, intravenous pentamidine should be used instead. Pentamidine-related toxicities – acute pancreatitis, hypoglycemia, and renal failure – are, however, common and should be aggressively sought when using this drug. Adjunctive steroids have been shown to improve survival in individuals presenting with a PaO2 ≤70 mm Hg or alveolar–arterial (A-a) gradients >30 mm Hg. Nonetheless, overall mortality rates, even in the post-HAART era, approach 30% and remain as high as 60% to 80% in ventilated patients.
| Pathogen | First-line treatment | Notes |
|---|---|---|
| Pneumocystis jirovecii | Mild to moderate disease: TMP–SMX-DS, 2 tabs PO TID; dapsone, 100 mg PO qd, plus TMP, 5 mg/kg PO q8h; clindamycin, 450 mg PO or 600 mg IV q8h, plus primaquine, 15 mg qd; or atovaquone, 1500 mg qd for 21 d Severe disease: TMP–SMX, 15 mg/kg IV (TMP component) qd in 3–4 divided doses, or, in patients with sulfa allergy, pentamidine, 4 mg/kg IV qd, plus prednisone (40 mg BID × 5 d; 20 mg BID × 5 d; 20 mg qd × 11 d) for 21 d | (1) Caution must be taken with the use of primaquine or dapsone in patients at risk for G6PD deficiency because of the risk of hemolytic anemia. Such individuals should be tested for the latter in anticipation of exposure to these drugs. (2) May discontinue secondary prophylaxis after CD4 >200 cells/mm3 for >3 mo |
| M. tuberculosis | Isoniazid, 5 mg/kg/d, plus rifampin, 600 mg PO qd, plus pyrazinamide, 15–30 mg/kg PO qd, plus ethambutol, 15–25 mg/kg/d as initial therapy | If patient is taking a protease inhibitor, replace rifampin with rifabutin, 150–300 mg PO qd, because rifampin significantly lowers serum levels of protease inhibitors |
| M. avium complex | Clarithromycin, 500 mg PO BID, or azithromycin, 600 mg qd, plus ethambutol, 15–25 mg/kg/d, plus rifabutin, 300 mg PO qd for a minimum of 12 mo | In patients on efavirenz, azithromycin is preferred over clarithromycin as part of the 3-drug regimen because efavirenz lowers the serum levels of the latter |
| M. kansasii | Isoniazid, 5 mg/kg/d, plus rifampin, 600 mg PO qd, plus ethambutol, 25 mg/kg/d (× 2 months then 15 mg/kg) for 15–18 mo | If patient is taking a protease inhibitor, replace rifampin with rifabutin, 150–300 mg PO qd, because rifampin significantly lowers serum levels of protease inhibitors |
| M. abscessus | Amikacin plus either cefoxitin or imipenem plus macrolide for 4–8 wk, followed by macrolide plus second agent for 6–12 mo | Monitor for inducible macrolide resistance |
| M. fortuitum | Amikacin or tobramycin plus 2 of cefoxitin or imipenem or levofloxacin for 2–6 wk followed by 2 oral agents for 12 mo | |
| Histoplasma capsulatum (pulmonary and non-CNS disseminated disease) | Mild-to-moderate disease: itraconazole, 200 mg PO TID for 3 d, followed by itraconazole, 200 mg BID for 12 wk Severe illness: amphotericin B deoxycholate, 0.7 mg/kg IV qd, or liposomal or lipid complex amphotericin, 3–5 mg/kg, until clinically improved followed by itraconazole, 200 mg q12h for 12 wk | |
| Coccidioides immitis (pulmonary and non-CNS disseminated disease) | Mild-to-moderate disease: fluconazole, 400–800 mg PO qd Severe illness: amphotericin B deoxycholate, 0.5–1.0 mg/kg IV qd, followed by fluconazole, 400–800 mg PO qd | Lifelong suppression with fluconazole, 400 mg PO qd, is recommended, even with CD4 >200 cells/mm3 HAART |
| Blastomycosis dermatitidis (pulmonary and non-CNS disseminated disease) | Amphotericin B deoxycholate, 0.7–1.0 mg/kg IV qd, or liposomal amphotericin, 3–5 mg/kg, until clinically improved, followed by itraconazole, 200 mg for 12 wk | |
| Aspergillus fumigatus (pulmonary and non-CNS disseminated disease) | Voriconazole, 6 mg/kg IV × 1, followed by either 4 mg/kg IV or 100–200 mg PO BID for 2–3 wk; oral voriconazole for suppression | (1) Alternatives to voriconazole: amphotericin B deoxycholate; liposomal or lipid complex amphotericin 3–5 mg/kg; or caspofungin +/− voriconazole (2) Also note: when using voriconazole with efavirenz, voriconazole dose should be increased to 400 mg q12h and decrease the efavirenz to 300 mg QD |
| Nocardia asteroides (pulmonary and non-CNS disseminated disease) | TMP–SMX (15 mg/kg/d TMP; 75 mg/kg/d SMX) × 3 wk followed by 10 mg/kg/d TMP component PO | In severe cases: add amikacin or imipenem or third-generation cephalosporin when limited by aminoglycoside toxicity |
| Rhodococcus equi (pulmonary and non-CNS disseminated disease) | Erythromycin or imipenem, 0.5 g IV q6h, plus rifampin, 600 mg PO qd for 2 wk, followed by oral clarithromycin or azithromycin plus rifampin for suppression | Alternative agents: ciprofloxacin or linezolid |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree