Sustained clinical cytopenia is a frequent laboratory finding in ambulatory and hospitalized patients. For pathologists and hematopathologists who examine the bone marrow (BM), a diagnosis of cytopenia secondary to an infiltrative BM process or acute leukemia can be readily established based on morphologic evaluation and flow cytometry immunophenotyping. However, it can be more challenging to establish a diagnosis of myelodysplastic syndrome (MDS). In this article, the practical approaches for establishing or excluding a diagnosis of MDS (especially low-grade MDS) in patients with clinical cytopenia are discussed along with the current diagnostic recommendations provided by the World Health Organization and the International Working Group for MDS.
Overview
- 1.
Myelodysplastic syndromes (MDS) are hematopoietic stem cell neoplasms that involve the blood and bone marrow (BM) and manifest with varying degrees of peripheral cytopenias and morphologic dysplasia.
- 2.
The BM in MDS is often hypercellular with disturbed topography, but 15% of cases are hypocellular.
- 3.
Morphologic dysplasia must be present in more than 10% of cells in at least one hematopoietic lineage; in the absence of significant dysplasia, MDS can only be diagnosed if characteristic cytogenetic abnormalities are present in the clinical setting of unremitting cytopenia.
- 4.
Accurate enumeration of myeloblasts in MDS is critical for classification and risk stratification.
- 5.
Flow cytometry immunophenotyping can be useful in differentiating a reactive process versus a neoplastic stem cell process, such as MDS.
Sustained (≥6 months) clinical cytopenia involving one or more hematopoietic lineages (erythrocytes, neutrophils, or platelets) is a frequent laboratory finding in patients treated at ambulatory clinics and in hospitalized patients. The first question to be addressed (usually by a clinician, before considering sampling the BM) is whether or not the cytopenia is due to decreased production of hematopoietic cells or increased destruction, consumption, or loss of blood cells. For cytopenia attributed to decreased production of hematopoietic cells, the underlying causes can be attributed to either an intrinsic hematopoietic stem cell disorder or secondary etiology. Increased destruction or consumption can be due to blood loss, hemolysis, hypersplenism, mechanical cardiac valve placement, and other factors. The current recommended guidelines for the initial clinical evaluation of patients with cytopenia generally include a review of the patient’s medical history and a thorough laboratory work-up (complete blood count and assessment of iron, folate, and vitamin B 12 levels). BM biopsies and aspirates are subsequently performed in some cases because of an unrevealing laboratory work-up or to rule out an infiltrative BM process.
For pathologists who examine the BM, a diagnosis of cytopenia secondary to an infiltrative process (such as lymphoma, myeloma, or metastatic carcinoma) or an acute leukemia can usually be easily established based on morphologic evaluation and flow cytometry immunophenotyping (FCI). It can be more challenging, however, to establish a diagnosis of MDS, a clonal BM disorder characterized by peripheral cytopenia, ineffective hematopoiesis, morphologic dysplasia, and recurrent cytogenetic abnormalities. Traditionally, the gold standard for diagnosing MDS has been BM morphology and cytogenetic studies in conjunction with a clinical presentation of persistent and unexplained cytopenia. Not all patients with clinically suspected MDS evince definitive morphologic dysplasia, and some non-MDS-related cytopenias may mimic MDS on morphologic evaluation. Moreover, cytogenetic abnormalities are infrequent in patients with MDS cases lacking excess blasts. The 2008 World Health Organization (WHO) classification subcategorizes MDS into several different diseases, based on the types of cytopenias, morphology, and specific cytogenetic abnormalities. These disease categories have different clinical behaviors and prognosis. The MDS entities defined in the 2008 WHO classification and their abbreviations are listed in Box 1 . Although there is no recognized pathologic grading scheme for MDS, for the purposes of this topic, lower-grade MDS cases are defined as blasts greater than 5%, including refractory cytopenia with unilineage dysplasia (RCUD), refractory cytopenia with multilineage dysplasia (RCMD), and MDS with del(5q), whereas higher-grade MDS cases are defined as blast greater than or equal to 5%, including refractory anemia with excess blasts (RAEB).
Refractory cytopenia with unilineage dysplasia (RCUD)
Refractory neutropenia
Refractory anemia
Refractory thrombocytopenia
Refractory anemia with ring sideroblasts (RARS)
Refractory cytopenia with multilineage dysplasia (RCMD)
Refractory anemia with excess blasts (RAEB)
RAEB-1
RAEB-2
MDS with isolated del(5q) abnormality
MDS, unclassified
Therapy-related MDS (t-MDS)
Diagnosing MDS can be particularly challenging in patients who have received chemotherapy for primary malignancies and developed prolonged cytopenia. In such patients, a question to answer is whether the cytopenia is attributed to BM injury due to exogenous factors or if MDS has developed secondary to the chemotherapy. Morphologic dysplasia is a frequent finding in postchemotherapy BM samples and becomes less reliable in making the distinction between MDS and postchemotherapy BM injury. A similarly challenging situation exists in the differential diagnosis between aplastic anemia (AA) and hypoplastic MDS cases. Although the BM of patients with AA exhibits hypocellularity and usually lacks significant dysplasia, in some cases the distinction between hypocellular MDS and AA may not be possible. Morphologic evaluation can be further hampered by suboptimal BM material. For example, core biopsy samples may be of inadequate length, consist predominantly of cortical bone, or exhibit obscuring crush artifact. In addition, aspirate smears may be inadequate because of hemodilution, air drying, or poor staining.
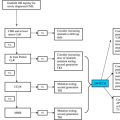
This content addresses the diagnostic challenges faced by pathologists interpreting BM samples taken to evaluate cytopenic patients. The morphologic and clinical features that distinguish MDS from cytopenias secondary to non-MDS causes are described, in particular, the appropriate interpretation of cytogenetic findings and application of ancillary testing (mainly FCI). An algorithm based on the WHO and International Working Group (IWG) guidelines for diagnosis of MDS is also provided.
Clinical features
As defined by the International Prognostic Scoring System (IPSS) for MDS, cytopenia is characterized by a hemoglobin level less than 10 g/dL, absolute neutrophil count less than 1.8 × 10 9 /L, and platelet count less than 100 × 10 9 /L. The IWG defines cytopenia as a hemoglobin level less than 11 g/dL, absolute neutrophil count less than 1.5 × 10 9 /L, and platelet count less than 100 × 10 9 /L. Cytopenia in MDS is often unremitting or progressive. In some patients, however, cytopenia can be less severe at presentation or have less than a 6-month duration due to early detection. A diagnosis of MDS can still be rendered in such settings if definitive morphologic and/or cytogenetic findings are present ( Box 2 ).
- A.
Prerequisite criteria (both 1 and 2 required)
- 1.
Constant cytopenia in one or more of the following cell lineages:
- •
Erythroid (hemoglobin <11 g/dL a )
- •
Neutrophilic (absolute neutrophil count <1.5 × 10 9 /L b ) or
- •
Megakaryocytic (platelets <100 × 10 9 /L)
- •
- 2.
Exclusion of all other hematopoietic or nonhematopoietic disorders as the primary reason for cytopenia/dysplasia
- 1.
- B.
MDS-related decisive criteria (at least one required)
- •
Dysplasia in ≥10% of all cells in at least one of the following lineages in the BM smear: erythroid, neutrophilic, or megakaryocytic, or >15% ringed sideroblasts (iron stain)
- •
5%–19% Blast cells in the BM or PB
- •
Typical chromosomal abnormality (by conventional karyotyping or fluorescence in situ hybridization [FISH])
- •
- C.
Co-criteria (for patients fulfilling “A” but not any of the “B” criteria above and who otherwise show typical clinical features [eg, transfusion-dependent macrocytic anemia]) (at least one required)
- •
Abnormal phenotype of BM cells clearly indicative of a monoclonal population of erythroid and/or myeloid cells, determined by flow cytometry,
- •
Clear molecular signs of a monoclonal cell population on X-inactivation assay, gene chip profiling, or point mutation analysis (eg, RAS mutations),
- •
Markedly and persistently reduced colony formation (±cluster formation) of BM and/or circulating progenitor cells by colony-forming unit assay.
- •
a The WHO 2008 classification recommends using a hemoglobin level of <10 g/dL.
b The WHO 2008 classification recommends using an absolute neutrophil count level of <1.8 × 10 9 /L.
Most patients with MDS present with cytopenia-related symptoms. Symptoms related to anemia, such as fatigue and malaise, with eventual transfusion dependence, are most common. Patients can also present with petechiae, ecchymoses, and nose and gum bleeding due to thrombocytopenia. Fever, cough, or septic shock may be manifestations of serious bacterial or fungal infections secondary to neutropenia. Hepatosplenomegaly or lymphadenopathy is uncommon in MDS patients. Therapy-related MDS (t-MDS) and other therapy-related myeloid neoplasms secondary to alkylating agents and/or ionizing radiation most commonly occur 5 to 10 years after exposure. Patients often present with t-MDS and evidence of BM failure, although a minority may present with t-MDS/myeloproliferative neoplasm (MPN) or with overt therapy-related acute myeloid leukemia (AML). Therapy-related myeloid neoplasms after treatment with topoisomerase II inhibitors have a latency period of approximately 1 to 5 years; most of these patients do not develop MDS but instead present with overt AML. In practice, many patients have received chemotherapeutic regimens that include both alkylating agents and topoisomerase II inhibitors and the clinicopathologic features may not be clear-cut. In addition, in elderly patients who receive cytotoxic therapy, cytopenias may be due to coincidental primary MDS or concurrent MDS due to genetic predisposition to cancer. Nevertheless, because they are difficult or impossible to distinguish from t-MDS, according to the WHO classification such cases are considered by default to represent t-MDS.
Clinical features
As defined by the International Prognostic Scoring System (IPSS) for MDS, cytopenia is characterized by a hemoglobin level less than 10 g/dL, absolute neutrophil count less than 1.8 × 10 9 /L, and platelet count less than 100 × 10 9 /L. The IWG defines cytopenia as a hemoglobin level less than 11 g/dL, absolute neutrophil count less than 1.5 × 10 9 /L, and platelet count less than 100 × 10 9 /L. Cytopenia in MDS is often unremitting or progressive. In some patients, however, cytopenia can be less severe at presentation or have less than a 6-month duration due to early detection. A diagnosis of MDS can still be rendered in such settings if definitive morphologic and/or cytogenetic findings are present ( Box 2 ).
- A.
Prerequisite criteria (both 1 and 2 required)
- 1.
Constant cytopenia in one or more of the following cell lineages:
- •
Erythroid (hemoglobin <11 g/dL a )
- •
Neutrophilic (absolute neutrophil count <1.5 × 10 9 /L b ) or
- •
Megakaryocytic (platelets <100 × 10 9 /L)
- •
- 2.
Exclusion of all other hematopoietic or nonhematopoietic disorders as the primary reason for cytopenia/dysplasia
- 1.
- B.
MDS-related decisive criteria (at least one required)
- •
Dysplasia in ≥10% of all cells in at least one of the following lineages in the BM smear: erythroid, neutrophilic, or megakaryocytic, or >15% ringed sideroblasts (iron stain)
- •
5%–19% Blast cells in the BM or PB
- •
Typical chromosomal abnormality (by conventional karyotyping or fluorescence in situ hybridization [FISH])
- •
- C.
Co-criteria (for patients fulfilling “A” but not any of the “B” criteria above and who otherwise show typical clinical features [eg, transfusion-dependent macrocytic anemia]) (at least one required)
- •
Abnormal phenotype of BM cells clearly indicative of a monoclonal population of erythroid and/or myeloid cells, determined by flow cytometry,
- •
Clear molecular signs of a monoclonal cell population on X-inactivation assay, gene chip profiling, or point mutation analysis (eg, RAS mutations),
- •
Markedly and persistently reduced colony formation (±cluster formation) of BM and/or circulating progenitor cells by colony-forming unit assay.
- •
a The WHO 2008 classification recommends using a hemoglobin level of <10 g/dL.
b The WHO 2008 classification recommends using an absolute neutrophil count level of <1.8 × 10 9 /L.
Most patients with MDS present with cytopenia-related symptoms. Symptoms related to anemia, such as fatigue and malaise, with eventual transfusion dependence, are most common. Patients can also present with petechiae, ecchymoses, and nose and gum bleeding due to thrombocytopenia. Fever, cough, or septic shock may be manifestations of serious bacterial or fungal infections secondary to neutropenia. Hepatosplenomegaly or lymphadenopathy is uncommon in MDS patients. Therapy-related MDS (t-MDS) and other therapy-related myeloid neoplasms secondary to alkylating agents and/or ionizing radiation most commonly occur 5 to 10 years after exposure. Patients often present with t-MDS and evidence of BM failure, although a minority may present with t-MDS/myeloproliferative neoplasm (MPN) or with overt therapy-related acute myeloid leukemia (AML). Therapy-related myeloid neoplasms after treatment with topoisomerase II inhibitors have a latency period of approximately 1 to 5 years; most of these patients do not develop MDS but instead present with overt AML. In practice, many patients have received chemotherapeutic regimens that include both alkylating agents and topoisomerase II inhibitors and the clinicopathologic features may not be clear-cut. In addition, in elderly patients who receive cytotoxic therapy, cytopenias may be due to coincidental primary MDS or concurrent MDS due to genetic predisposition to cancer. Nevertheless, because they are difficult or impossible to distinguish from t-MDS, according to the WHO classification such cases are considered by default to represent t-MDS.
Diagnosis: microscopic features
Peripheral Blood
The peripheral blood (PB) in patients with MDS nearly always shows some evidence of cytopenia. In addition, the anemia is often macrocytic and the red cell distribution width is often increased. Patients with significant ring sideroblasts (RS) in the marrow may show a dimorphic red cell appearance due to a combination of macrocytes and hypochromic microcytes. Granulocytic dysplasia may be more visible in the PB than in the BM. Recognizing and reporting circulating blasts are important, because the presence of these blasts can change the disease classification and predict patients’ clinical outcomes. Circulating immature cells, including blasts, can also be seen in patients who have received growth factor treatment, in those with an actively regenerating BM, in those with an acute marrow stress such as sepsis, or in those who have undergone recent stem cell transplantation.
Bone Marrow Biopsy
Bone marrow biopsy is important for assessing cellularity, relative lineage proportions, fibrosis, stromal alterations, and megakaryocytic dysplasia. Normal BM usually shows a cellularity appropriate for a patient’s age, with orderly maturation and a normal cellular distribution: the myeloid precursors are generally found along the bone trabeculae, whereas the erythroid and megakaryocytic precursors are located more centrally ( Fig. 1 A). In MDS, the BM is usually hypercellular, and the BM topography is disrupted (see Fig. 1 B). Altered stroma is common, including markedly uneven distribution of fat cells with alteration of adipocyte size, increased histiocytes, increased small blood vessels, and increased reticulin fibrosis. Significant differences in the size of hemopoietic islands are often present and the erythroid islands may be disrupted or poorly delineated. Abnormal localization of immature precursors (ALIPs), defined as clusters or aggregates of myeloblasts and promyelocytes located away from bone trabeculae (see Fig. 1 C), is an adverse prognostic feature in MDS and is an uncommon finding in lower-grade MDS cases. However, ALIPs are not unique to MDS and can be seen in active BM regeneration or after growth factor treatment.
Immunohistochemistry (IHC) can be useful in assessing for MDS, especially in a case of fibrotic or hypocellular BM. The expression of CD34, a marker of early progenitor cells, is positive in most MDS blasts, regardless of the MDS subtype. The presence of increased and/or clustered CD34+ blasts not only helps confirm a diagnosis of MDS but also assists in identifying ALIPs that are correlated with increased risk of transformation to AML. CD34 is also present on endothelium and sinus lining cells, highlighting the increased angiogenesis characteristic of MDS. In rare cases, blasts in MDS are negative for CD34 and CD117 (c-KIT) may be used as an alternative blast marker; however, CD117 also stains some early erythroid precursors (pronormoblasts), some promyelocytes, and mast cells. Megakaryocytic markers, such as CD61, CD42b, and von Willebrand factor-associated protein, are useful for highlighting micromegakaryocytes and abnormal groupings or clusterings of megakaryocytes. These markers are also helpful in differentiating MDS from acute megakaryocytic leukemia (M7), where the megakaryoblasts have an immature phenotype, most commonly CD61+ but with partial or negative staining for CD42 and von Willebrand factor-associated protein.
Bone Marrow Aspirate
High-quality BM aspirate smears are critical for diagnosing and classifying MDS. BM aspirate evaluation includes recording the percentage of blasts and the degree of unilineage or multilineage dysplasia. Dysplasia must be present in at least 10% of the cells of any lineage, and the particular dysplastic changes seen may be relevant in predicting the biology and specific cytogenetic abnormalities of MDS.
Dysgranulopoiesis includes nuclear hypolobation, such as the pseudo-Pelger-Huët anomaly, hypersegmentation of the nuclei at an inappropriate stage, and abnormal cytoplasm ix granularity (agranularity, hypogranularity, hypergranularity or large, irregularly shaped eosinophilic pseudo-Chédiak-Higashi granules). Abnormally large or small granulocytes are also evidence of dysplasia, but giant neutrophils with nuclear hypersegmentation can also be seen in patients with vitamin B 12 or folate deficiency. Dysplastic features present at earlier stages of myeloid lineage include hypogranulation, abnormally shaped (eg, elongated) granules, abnormal nuclear lobation, and nuclear/cytoplasmic dyssynchrony ( Fig. 2 A, B). Recognization of dysplastic features in early myeloid cells is important, especially in cases of MDS with left-shifted myeloid maturation.
Dyserythropoietic features include nuclear budding, internuclear bridging, karyorrhexis, multinuclearity, nuclear hyperlobation, cytoplasmic basophilic stippling, and vacuoles. Megaloblastoid change (nuclear-cytoplasmic dyssynchrony) is nonspecific and is common in non-MDS BM samples, thus should not be overinterpreted. RS, another manifestation of dyserythropoiesis, should have at least five siderotic granules present, covering at least one-third of the circumference of the nucleus. Erythroblasts with less than five granules or with granules that are not in a perinuclear location should not be counted as RS. The presence of 15% RS or greater in a BM with less than 5% blasts classify patients as having refractory anemia with RS (RARS) if dysplasia is confined to the erythroid lineage. The presence of RS in MDS with multilineage dysplasia, cases with specific cytogenetic abnormalities, or those with increased blasts (5% or greater) do not change the MDS subcategorization. RS may occur in non-neoplastic conditions, such as alcoholism, hereditary sideroblastic anemia, and due to effects of certain drugs.
Marked erythroid hyperplasia (50% or greater) with or without left-shifted erythroid maturation (see Fig. 2 C) can be seen in approximately 15% of patients with MDS. RS are frequently present (see Fig. 2 D). In these patients, myeloblasts should be assessed as a proportion of nonerythroid cells: if myeloblasts are 20% or greater of nonerythroid cells, the case would meet criteria for acute erythroid leukemia, erythroid/myeloid subtype (FAB: AML-M6A); if myeloblasts are less than 20% of nonerythroid cells, the current recommendation is to enumerate the blasts as a proportion of total cells for subcategorization. Recent studies have shown that in erythroid-predominant MDS, however, blast calculation as a proportion of BM nonerythroid cells may be better than total nucleated cells for stratifying patients into prognostically relevant groups, and MDS with erythroid predominance and M6A may be a biologic continuum that is arbitrarily divided by a blast cut-off. Conversely, erythroid hypoplasia or aplasia is observed in approximately 5% of MDS cases (see Fig. 2 E, F) and is often associated with an oligo- or monoclonal T-cell proliferation, suggesting immune-mediated destruction of erythroid precursors. Some of these patients may respond to cyclosporine treatment.
Dysmegakaryopoiesis in MDS is characterized by micromegakaryocytes with hypolobated nuclei; megakaryocytes of all sizes with monolobated nuclei; or megakaryocytes with multiple, widely separated nuclei (pawn-ball appearance). However, the latter forms can be seen in non-MDS conditions, such as paraneoplastic syndrome. Megakaryocytes can be increased, decreased, or normal in number. In evaluating megakaryocyte dysplasia, the best approach is to make the initial evaluation based on BM biopsy and then verify the findings on the BM aspirate smears. Commenting on dysmegakaryopoiesis should be based on an assessment of at least 20 to 30 megakaryocytes, ideally including evaluation of megakaryocytes in both the biopsy sections and aspirate smears.
Blast recognition and enumeration are critical in the diagnosis, risk stratification, and assessment of treatment response in MDS. A 500-cell differential count is required for accurate blast enumeration. The presence of Auer rods shifts the classification to RAEB-2, regardless of the blast percentage. Myeloblasts in MDS often show marked heterogeneity in size and can be classified into two morphologic types: agranular and granular. Promyelocytes in MDS can be misinterpreted as granular blasts due to dysplastic changes. Normal promyelocytes have a visible Golgi zone, uniformly dispersed azurophilic granules, and, in most instances, basophilic cytoplasm, whereas dysplastic promyelocytes may have reduced or irregular cytoplasmic basophilia, a poorly developed Golgi zone, hyper- or hypogranularity, or irregular distribution (clumps) of granules. Unlike most blasts in MDS, however, dysplastic promyelocytes should still contain an oval or indented nucleus that is often eccentric with somewhat coarse chromatin and at least a faintly visible Golgi zone. Dysplastic promyelocytes are also often larger than myeloblasts. Some myeloblasts can have deeply basophilic cytoplasm and can be confused with early erythroid precursors ( Fig. 3 ). Erythroid precursors have relatively mature clumped chromatin and often larger than myeloblasts at early stages.
Diagnosis: ancillary studies
Flow Cytometric Immunophenotyping
Flow cytometric immunophenotyping (FCI) is a highly sensitive and reproducible method for quantitatively and qualitatively evaluating hematopoietic cell abnormalities. FCI abnormalities in MDS have been shown to be highly correlated with morphologic dysplasia and cytogenetic abnormalities. In addition, FCI is less subjective and may be more sensitive and less affected by specimen quality than morphologic evaluation of dysplasia on smears. In particular, FCI demonstrates usefulness in supporting or ruling out MDS in the most diagnostically challenging BM samples obtained from patients with chronic, persistent cytopenia with no significant morphologic dysplasia or cytogenetic abnormalities. A positive FCI result is more indicative of MDS or MDS/MPN whereas a negative FCI result is more frequently associated with non-MDS-related cytopenia. The use of FCI as an ancillary test in diagnosing MDS is gradually gaining acceptance by most pathologists. Given the wide range of findings observed in MDS thus far, however, FCI is only recommended for experienced laboratories, because some of the changes seen in FCI overlap with the changes seen in reactive and recovering BM samples.
Recently, the European LeukemiaNet has published standardization of FCI in diagnosis of MDS, providing guidelines on panel design and data interpretation. Most published studies using FCI for diagnosing MDS are based on interpreting altered myelomonocytic differentiation or maturation patterns, using antigen combinations, such as CD13/CD16, CD11b/CD16, CD64/CD10, CD33/HLA-DR, CD65, and CD15. These approaches are sensitive, and MDS cases have demonstrated multiple abnormalities on FCI. However, myelomonocytic maturation patterns on FCI can show alterations in patients with reactive conditions, such as in patients with regenerating BM, those who have received growth factor treatment, and those with acute BM injury, severe infection, HIV, or autoimmune conditions. In addition, specimen quality such as hemodilution, or aged samples as well as increased eosinophils, can alter some normal patterns and the expression levels of some markers (eg, CD16, CD11b, and CD10). Moreover, abnormalities of some markers, such as decreased CD33 expression, can be attributed to genetic polymorphism and are not necessarily indicative of dysplasia. Although these nonspecific changes can be recognized, the pattern recognition methodology requires that pathologists have extensive knowledge and experience in normal myelomonocytic maturation patterns and understand the immunuphenotypic mimics.
In contrast, a focus on myeloblast phenotype seems to be a better approach. Changes in myeloblasts more reliably indicate a stem cell neoplasm and are uncommonly seen in reactive conditions. These findings are easier for pathologists to interpret and report. This type of FCI analytic approach is focused on CD34+ cells, which in a reactive BM should show diverse differentiation and maturation patterns ( Fig. 4 ). On side scatter versus CD45, normal myeloid precursors are scattered, showing a normal level of CD45 expression (often at the same level of granulocytes and side scatter). The normal CD34+ stem cells are able to produce hematogones (normal CD19+, CD10+ immature B-cell precursors), plasmacytoid dendritic precursors (CD123bright+, HLA-DR+), differentiating myeloid precursors (CD34+, CD15+, CD65+), and monocytic precursors (CD34+, CD64+, CD4+). In contrast, the CD34+ blasts in MDS appear to be clonal, forming a discrete population on side scatter versus CD45 and lacking evidence of differentiation toward hematogones, plasmacytoid dendritic cells, or monocytes. Alterations of antigenic expression levels, such as decreased or increased CD45 expression, increased CD34 or CD117 expression (see Fig. 4 ), increased CD33 or CD13 expression, or decreased CD38 expression, are often observed. Aberrant expression of lymphoid antigens (eg, CD2, CD5, CD7, and CD56) can also be observed.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree