Decreased platelet production (hypoplastic thrombocytopenia)
Aplastic anemia (AA)
Amegakaryocytic thrombocytopenia
Acute leukemia
Chemotherapy-induced thrombocytopenia (CIT)
Myelodysplastic syndrome (MDS)
Malignancy with bone marrow infiltration or suppression (e.g., lymphoma, leukemia, solid tumors)
Increased platelet destruction or consumption
Autoimmune thrombocytopenia (ITP)
Secondary ITP
Systemic lupus erythematosus (SLE)
Drug-induced immune thrombocytopenia
Infection associated (e.g., HIV, HCV, H. pylori, EBV)
Thrombotic microangiopathy
Thrombotic thrombocytopenic purpura (TTP)
Hemolytic uremic syndrome (HUS)
Disseminated intravascular coagulation (DIC)
Antiphospholipid syndrome (APS)
2 Diagnostic Markers for the Differentiation Between ITP and Other Thrombocytopenic Disorders Due to Increased Platelet Destruction or Consumption
Before discussing differential diagnosis between ITP and hypoplastic thrombocytopenia, differentiation between ITP and other thrombocytopenic disorders due to increased platelet destruction or consumption should be discussed. As shown in Table 2, there are several diagnostic markers available to distinguish other thrombotic disorders from ITP. Schistocytes (=RBC fragments) in peripheral blood smear suggest thrombotic microangiopathy such as thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS) [5]. Thus, as compared with differential diagnosis with hypoplastic thrombocytopenia, the differentiation between ITP and other thrombocytopenic disorders due to increased platelet destruction or consumption is not so difficult in daily practice.
Table 2
Diagnostic markers for thrombocytopenia due to increased platelet destruction or consumption
Secondary ITP |
Systemic lupus erythematosus (SLE): antinuclear antibodies (ANA) (anti-double-stranded DNA [dsDNA] and anti-Sm) |
Drug-induced immune thrombocytopenia: history of medication |
Infection associated: tests for HIV, HCV, H. pylori, EBV, etc. |
Thrombotic microangiopathy: microangiopathic hemolytic anemia (MAHA), schistocytes (RBC fragments) |
TTP: ADAMTS13 activity, inhibitor for ADAMTS13 |
HUS: detection of Shiga toxin-producing Escherichia coli (STEC) |
DIC: screening tests of coagulation (prothrombin time [PT], activated partial thromboplastin time [aPTT], fibrinogen, and D-dimer) |
APS: antiphospholipid antibodies (anticardiolipin antibody, anti-β2-glycoprotein I antibody, lupus anticoagulant) |
3 Diagnostic Markers for the Differentiation Between ITP and Hypoplastic Thrombocytopenia
3.1 Bone Marrow Examination
The need for bone marrow examination is still a matter of debate. The current American Society of Hematology (ASH) ITP guideline 2011 does not recommend a bone marrow examination in patients presenting with typical ITP, although the previous ASH guideline 1996 and international working group (IWG) consensus report recommended bone marrow examination for patients older than 60 years of age, for those with systemic symptoms or abnormal signs, or for some cases in which splenectomy is considered [4, 6, 7]. AA showing pancytopenia is easy for its diagnosis. However, mild AA with thrombocytopenia is often difficult for its diagnosis even by bone marrow examination (bone marrow aspiration and bone marrow biopsy). For considering the limitation of this procedure and to avoid this invasive procedure as initial screening, we need diagnostic markers for the differential diagnosis between ITP and hypoplastic thrombocytopenia.
3.2 Platelet-Associated IgG (PAIgG)
Several assays to detect antiplatelet antibodies as platelet-associated IgG (PAIgG) on platelets have been reported in the 1970s [1]. However, elevated levels of PAIgG were not specific for ITP but were often observed in patients with nonimmune thrombocytopenia including AA [8, 9]. IWG classified PAIgG as a test of uncertain benefit for the diagnosis of ITP [4]. More detailed nature of PAIgG is described in the chapter “Autoantigens in ITP.”
3.3 Platelet-Associated Antiplatelet Autoantibodies
Platelet-associated (PA) anti-GPIIb/IIIa and/or anti-GPIb-IX autoantibodies were detected in 50–60% ITP patients but not in patients with aplastic anemia [10, 11] (refer to the chapter “Autoantigens in ITP”). IWG classified PA antiplatelet autoantibodies (=glycoprotein-specific antibody) as a test of potential utility in the management of an ITP patient [4]. Regarding detection of PA autoantibodies, it has been shown that its specificity for the diagnosis of ITP is very high (80–90%) in prospective studies. However, the drawback in this assay is its relatively low sensitivity (50–60%) as well as being time-consuming, laboratory-based assay such as the immunobead assay and monoclonal antibody immobilization of platelet antigens (MAIPA) [11, 12]. Thus, from a clinical view point, it is difficult to perform this specific assay as a screening test for the diagnosis of ITP.
3.4 Plasma Thrombopoietin (TPO) Level and Percentage of Reticulated Platelets (RP%)
IWG consensus report classified plasma TPO level and RP% as tests of unproven or uncertain benefit for the diagnosis of ITP in 2010 [4]. In contrast, Japanese Working Group for ITP suggested that these markers may be useful for the diagnosis prospectively as well as retrospectively [13, 14]. To date, there are a substantial number of papers showing the usefulness of these markers as differential diagnosis of ITP.
Plasma TPO Level
In 1994, three groups independently cloned TPO, as the ligand for orphan cytokine receptor, c-Mpl [15–17]. TPO is a single 95 kDa glycoprotein consisting of 332-amino acid protein that is the primary regulator of normal platelet production. TPO is synthesized primarily in the liver and is secreted into circulation. Although it has long been thought that hepatic transcription and translation of the TPO gene appear constant, recent study reveals that TPO production is stimulated, at least in part, by desialylated, senescent platelets clearance via hepatocyte Ashwell-Morrell receptor. Hepatocyte Ashwell-Morrell receptor regulates TPO production via JAK2-STAT3 signaling [18, 19]. Secreted TPO is removed from circulation by binding of the TPO receptor (c-Mpl) on platelets and bone marrow megakaryocytes. Thus, plasma TPO levels usually increase in response to the decreased in platelet/megakaryocyte mass. Actually, plasma TPO levels are markedly increased in patients with congenital amegakaryocytic thrombocytopenia, aplastic anemia, or chemotherapy-induced thrombocytopenia (CIT). In sharp contrast, in patients with ITP (or thrombocytopenia due to increased platelet destruction or consumption), plasma TPO levels are within normal range or slightly increased [20–23]. Thus, plasma TPO levels may have diagnostic utility in discriminating between patients with ITP and hypoplastic thrombocytopenia [13, 14, 24].
RP%
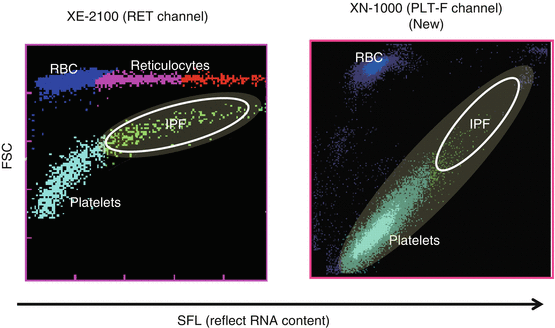
Reticulated platelets (RPs) are reported to be younger platelets (i.e., immature platelets) that have been released recently into the circulation and are probably analogous to reticulocytes reflecting erythropoiesis. RPs can be distinguished from mature platelets by their RNA contents using flow cytometry with an RNA-binding fluorochrome, such as thiazole orange, and RP% and absolute number of RPs are reflecting platelet production and hence platelet turnover [25–27]. In patients with ITP, RP% was markedly increased compared with healthy controls, whereas RP% in patients with AA or CIT was within normal range [13, 25, 26]. Thus, RP% could also be a good marker to differentiate ITP from hypoplastic thrombocytopenia. However, the method for the measurement of RP% is a time-consuming, laboratory-based assay and has not been standardized yet, because RP% is measured by flow cytometry. To measure RP% in daily practice, percentage of immature platelet fraction (IPF%) could alternatively be measured by automated hematology analyzer such as Sysmex® XN-1000. As compared to XE-2100 (Sysmex Corp., Kobe, Japan), XN-1000 is a newer generation that uses oxazine to detect RNA and PLT-F channel to more accurately detect platelets (Fig. 1). We have compared RP%, IPF% measured by XE-2100, and IPF% measure by XN-1000 (newer generation) in parallel to differentiate patients with ITP from aplastic anemia or CIT who were already well diagnosed. The sensitivity and specificity for the diagnosis of ITP were 83.0 and 75.0% for IPF% (XE), 85.1 and 89.3% for IPF% (XN), and 93.6 and 89.3% for RP%, respectively. Moreover, examination of patients with paroxysmal nocturnal hemoglobinuria (PNH) revealed that hemolysis and/or red blood cell fragments interfered with IPF% (XE) values, but not with RP% or IFP% (XN) values [28]. Thus, IPF% measured by XN-1000 appears comparable to RP% measured by flow cytometry and may have diagnostic utility in discriminating between patients with ITP and hypoplastic thrombocytopenia in daily practice.