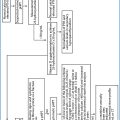
Corrected total calcium or ionized calcium
Both elevated or only free calcium
Second or third PTH generation assays
High or inappropriately normal
25-OH-vitamin D
The deficit should be corrected to values
of 20-30 ng/mL
Urinary calcium excretion
Usually increased
7.1.1 PTH Assays
PTH is a protein composed of 84 amino acids. It circulates as a set of fragments but only the first 34 residues have biological activity.
First-generation PTH assays were developed in the 1960s and 1970s and measured the C-terminal PTH fragment (not biologically active) as well as the intact 1-84 PTH molecule [3]. The capacity of these assays to distinguish non-parathyroid hypercalcemia from mild pHPT was limited because C-PTH production is stimulated by chronic hypercalcemia. Moreover, these fragments also circulate in higher concentrations in chronic renal failure, further complicating the interpretation of results.
Second-generation PTH assays (or intact PTH assays) initially became available in 1987 and they were more specific for intact (1-84) PTH molecule. However, recent critical evaluation has questioned their performance; indeed these assays react with relatively long fragments of PTH, which started their amino acid structure at position 4, 7, 10 and 15, of which the most abundant is PTH (7-84). These large amino-truncated PTH fragments represent 20% of the PTH immunoreactivity in healthy subjects but 50% in renal failure.
This has led to the recent development of a third-generation assay or “whole” PTH assay, which selectively recognizes only the 1-84 fragments. This assay was introduced in 1999 and it results are more specific for PTH (1-84) measurement, using a labeled antibody directed against PTH (1-4), and preventing cross-reactivity with PTH (7-84). However, the third-generation assay detects a post-translational modified form of PTH (1-84) in the region 15-20 by phosphorylation of a serine residue. This molecular form represents 10% of PTH values in healthy patients and 15% in renal failure, and it can be overproduced also in parathyroid carcinoma and severe HPT. Nevertheless, the mean PTH concentrations are lower with the third-generation assay than with the second one [4].
With regard to the diagnostic procedures of pHPT, second- and third-generation PTH assays show a similar diagnostic sensitivity, but they are better than the first-generation assay. Serum PTH reference intervals for each assay based on coexisting 25-hydroxyvitamin D (25-OH-D) levels are not yet available and further studies are required to establish them. An isolated value of increased PTH should be confirmed on at least two other times over a period of 3–6 months [5].
7.1.2 Calcium
Total calcium values include ionized free calcium (50% of the total), protein-bound calcium (40% of the total and 80% of this is associated with albumin) and calcium complexed with inorganic and organic anions such as phosphate, citrate and sulfate (10%) Only ionized calcium exerts biological actions [3].
Consequently, many factors of discrepancy between measurement of total and ionized calcium can be mentioned (Table 7.2):
Table 7.2
Causes of discrepancy between total and free calcium levels
Alteration of total calcium, | Alteration of free calcium, |
|---|---|
but not of free calcium | but not of total calcium |
Hypoalbuminemia | Acid-base disturbance |
Hemoconcentration and hemodilution | |
Paraproteinemia | |
Citrate-complexed calcium | |
Gadolinium | |
Mild hypercalcemia in pHPT |
Total calcium, but not free calcium, is increased by hemoconcentration (e.g. prolonged tourniquet use and dehydration) and is decreased by hemodilution (e.g. when blood sample is withdrawn on long term supine hospitalized patients) [6].
Hypoalbuminemia induces a reduction of total calcium, but not of ionized calcium. It is possible to adjust calcium for serum albumin concentration with textbook equations, for example:
![$$
\begin{array}{*{20}{c}}
{correctedserumcalcium = } \\
{serumcalcium(mg/dL) + 0.8x[4 - albumin(g/dL)].}
\end{array}
$$](http://oncohemakey.com/wp-content/uploads/2017/05/A978-88-470-5758-6_7_Chapter_TeX2GIF_Equa.gif)
These equations however are questionable, because they do not consider other factors that could affect the amounts of ionized, protein-bound or complexed calcium. Indeed, in addition to albumin, protein binding can be altered by the concentration of other proteins, abnormal proteins, pH, free fatty acids, bilirubin, drugs, and heparin [4]. Complex formation can be altered by variations in concentrations of citrate, phosphate, lactate, bicarbonate and other organic and inorganic anions [6].
Paraproteinemia (e.g. myeloma): abnormal globulins can bind calcium and affect total calcium measurement.
Excess soluble calcium chelating agents: total calcium can increase after transfusion of large quantities of citrated blood products; on the contrary, ionized calcium remains stable.
Gadolinium administration can decrease total calcium, but not ionized calcium.
Acid-base disturbance is the only condition causing problems in the interpretation of free calcium. Indeed acidosis decreases the binding between albumin and calcium, increasing the ionized component; alkalosis causes the contrary effect. If the patient shows acid-base disturbance the ionized calcium may be altered [3].
Mild hypercalcemia in pHPT: free calcium measurement appears to be a slightly better indicator of elevated calcium conditions than total calcium; thus, when a disorder of calcium metabolism is suspected, ionized calcium has increased sensitivity and specificity if compared with total calcium measurement, and in particular it is more sensitive for the detection of hypercalcemia in pHPT [6]. As a matter of fact, about 4–10% of patients with pHPT shows normal serum calcium with concomitant elevated ionized calcium [7].
The rationale for the highest diagnostic efficacy of free calcium in mild pHPT is not totally clear, because no changes in protein or albumin concentration or pH variations are observed [8].
In conclusion, the use of ionized calcium measurements should be encouraged, even if high interlaboratory variability limits the clinical usefulness [8].
7.1.3 25-Hydroxyvitamin D
Vitamin D circulates in three different forms: parent vitamin D and two metabolites. Calcidiol (25-OH-D) is produced in the liver and calcitriol (1,25-(OH)2-D3) is formed in the proximal kidney tubule. The preferred analyte to be measured in serum is 25-OH-D, as it has a longer half-life than parent vitamin D and circulates in nanomolar concentrations compared to 1,25-(OH)2-D3, which circulates in picomolar amounts [3].
The measurement of 25-OH-D concentrations is recommended in the diagnostic workup of pHPT and patients with vitamin D deficiency should be made carefully replete, with a minimal therapeutic target of 50 nmol/L (20 ng/mL), or better of 75 nmol/L (30 ng/mL). When 25-OH-D is repleted, a part of initially defined normocalcemic pHPT patients could become hypercalcemic and reclassified as hypercalcemic pHPT patients, previously masked by 25-OH-D deficiency [5]. Consequently, a more frequent monitoring of serum and urinary calcium is recommended when calciferol replacement takes place [3]. No additional information is obtained by 1,25-(OH)2-D3 measurement [5].
7.1.4 Other Laboratory Tests
Urinary calcium excretion is usually increased in pHPT. Urinary calcium concentrations >400 mg/24 result as a predictor of pHPT complications [5].
Serum creatinine and estimate glomerular filtration rate (eGFR) are useful to assess renal function and to correctly interpret PTH levels [5].
Calcium/creatinine clearance ratio is important to differentiate pHPT from familial hypocalciuric hypercalcemia (FHH), which is an autosomal dominant disorder characterized by asymptomatic and uncomplicated hypercalcemia, inappropriately normal PTH levels and relative hypocalciuria (see Section 7.3.5) [9].
Phosphate levels are frequently low in pHPT.
Chloride levels are usually high, leading to a mild hyperchloremic metabolic acidosis; bicarbonate levels are generally low for compensation.
7.2 Test to Confirm Primary Hyperparathyroidism
Some pHPT patients display either normal calcemia or normal plasma PTH levels with asymptomatic disease; in these cases, the diagnosis of pHPT is not easily established on basal data and it is important to determine whether these patients have early stage pHPT. Some authors reported that adenomatous parathyroid cells appear less sensitive to the negative feedback of calcium than normal cells [10–12] due to a decrease in the number of calcium-sensing receptors and to an alteration in the transduction system [13–16]. In order to differentiate pHPT from normal parathyroid function some authors suggest a PTH suppression test using i.v. calcium infusion [17–20], with good overall clinical tolerance. Both second- and third-generation PTH assays can be appropriately used. Different calcium infusion modalities and results interpretation were proposed obtaining good sensitivity and specificity values, but nevertheless able to differentiate pHPT patients from healthy controls. However, test execution modalities are not yet considered in the guidelines since they are not standardized and not properly supported by the literature.
Titon et al. [17] used 0.33 mmol/kg of 10% calcium gluconate added to 0.9% NaCl in order to obtain a total volume of 500 mL, which was infused in 3 hours; calcium, phosphorus and PTH were tested at basal time, at the end of calcium infusion and 3 hours later. In pHPT patients, PTH levels remained higher than 15 ng/L at the end of calcium loading (<15 ng/L in control subjects) and >26 ng/L 3 hours later (<20 ng/L in control subjects). Specificity was 93% and sensitivity 100% for the cut-off value of 12 ng/L; the values were 92% and 92%, respectively when a cut-off of 14 ng/L was considered.
Zhao et al. [18] used 4 mg/kg/h of calcium gluconate for 120 min and calcium, phosphorus and PTH were measured at baseline and after 30, 60, 90 and 120 min after start of infusion. PTH inhibition rate (PTH-IR; PTH reduction from baseline values) <73% at 120 min could optimally differentiate mild pHPT from normal subjects (sensitivity 95% and specificity 99.9%) even in the presence of vitamin D deficiency; furthermore, PTH-IR/∆Ca (∆Ca = increase in serum calcium levels from baseline) <1.27 had 90% in sensitivity and 82.4% in specificity.
As a whole, the calcium infusion test could represent a suitable tool for diagnostic confirmation in a subset of patients with normocalcemic pHPT or with inappropriate normal PTH. Before using it in the common clinical practice, a standardization of the infusion and a validation in a wider range of patients are required.
7.3 Differential Diagnosis
The diagnosis of pHPT should exclude other disorders characterized by secondary or compensatory increase of PTH with normal or increased calcium concentrations:
vitamin D deficiency
chronic kidney disease
drugs
hypercalciuria
hypocalciuric hypercalcemia
malabsorption syndromes.
7.3.1 Vitamin D Deficiency
An inverse relationship between serum 25-OH-D and PTH is well defined, thus, the insufficiency of vitamin D is generally associated with a slow increase in PTH, progressively rising when calcidiol concentration decreases below 110 nmol/L [21]. Vitamin D insufficiency is defined by 25-OH-D concentrations <50 nmol/L and vitamin D deficiency by concentrations <25 nmol/L [5]. A sufficient serum level of vitamin D can ensure ideal serum PTH values even when the calcium intake level is less than 800 mg/d [22]. The prevalence of vitamin D inadequacy is common, both in the general population and in patients with pHPT. The measurement of serum 25-OH-D levels is recommended in all patients with increased PTH levels and its careful correction is required in order to make the diagnosis of pHPT [5]. For other details, see Chapter 6.
7.3.2 Chronic Renal Failure
Secondary hyperparathyroidism is a common feature in patients with chronic kidney disease, due to an increased secretion of PTH because of a reduced synthesis of active vitamin D and the consequent calcium depletion with hyperphosphatemia. In addition to these mechanisms, some direct effects on parathyroid cells have also been shown, via the cell membrane calcium receptor, nuclear calcitriol receptor, and unknown cellular target of phosphate [23]. Thus, chronic renal failure not only leads to hormonal disturbances but also induces a parathyroid cell proliferation resulting in parathyroid hyperplasia. This condition may ultimately develop into an autonomous parathyroid function so, even if secondary hyperparathyroidism is corrected, parathyroid glands remain hyperfunctioning and this condition is called tertiary hyperparathyroidism. The clinical consequences of uremic secondary and tertiary hyperparathyroidism include renal osteodystrophy, calciphylaxis and potentially vascular calcifications. The higher morbidity and mortality mainly occurs with the increased cardiovascular risk. Population studies demonstrate that PTH begins to arise with eGFR <60 mL/min [5].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree