Diabetes Mellitus: Introduction
Diabetes mellitus is a common metabolic disorder affecting elderly people. Although it is recognized by its effects on carbohydrate metabolism to cause hyperglycemia, diabetes mellitus usually also affects lipid and protein metabolism. With time, effects of diabetes on the cardiovascular system, the kidneys, the retina, and the peripheral nervous system, often referred to as long-term complications of diabetes, substantially increase mortality and morbidity in older adults. Furthermore, diabetes may accelerate the risk and contribute to worse outcomes for other common age-related disorders. In general, diabetes mellitus in older adults is underdiagnosed and undertreated. A growing body of evidence assessing outcomes of interventions and an increasing number of therapeutic options for diabetes management has increased the importance of making a diagnosis and offering appropriate intervention strategies to elderly patients who have this potentially devastating disorder. There is also growing evidence of the ability to prevent the development of diabetes, including in older people. Management of diabetes is often considered to be synonymous with treatment of hyperglycemia. However, it is now recognized that appropriate diabetes management of an older patient must be much broader, addressing many factors that contribute to long-term complications.
Definition and Classification
Diabetes mellitus is a heterogeneous set of disorders affecting multiple body systems; however, diagnostic criteria are based on documentation of elevated circulating blood glucose levels. Because glucose levels vary during the course of the day, and even in the fasting state form a continuous variable in populations, the definition of a single cut point that separates normal from abnormal is somewhat arbitrary. The challenge of establishing appropriate diagnostic criteria for elderly subjects is made more difficult by well-described effects of aging on glucose metabolism (summarized later in this chapter in the section on “Effects of Aging”). Similar to criteria for hypercholesterolemia and hypertension, the diagnostic criteria for diabetes mellitus are based on values that predict poor outcomes in population studies.
Table 109-1 summarizes the currently accepted diagnostic criteria, which were established by an expert panel convened by the American Diabetes Association (ADA) and published in 1997 with a follow-up report in 2003.
Diabetes mellitus |
Classic diabetes symptoms plus a random glucose level ≥200 mg/dL (11.1 mmol/L) |
Fasting* glucose level ≥126 mg/dL (7.0 mmol/L)† |
Glucose level ≥200 mg/dL (11.1 mmol/L) at 2 hours during a standard OGTT† |
Impaired glucose tolerance (IGT) |
Glucose level ≥140 mg/dL (7.8 mmol/L) and <200 mg/dL (11.1 mmol/L) at 2 hours during a standard OGTT |
Impaired fasting glucose (IFG) |
Fasting glucose level ≥100 mg/dL (5.6 mmol/L) and <126 mg/dL (7.0 mmol/L) |
Type 1 diabetes is a condition characterized by destruction of the insulin-producing beta cells of the endocrine pancreas, resulting in absolute deficiency of insulin. While evidence of cell-mediated autoimmunity is a hallmark of type 1 diabetes, some patients develop a type 1 diabetes phenotype with evidence of severe insulin deficiency and episodes of diabetic ketoacidosis (DKA) with no detectable autoimmunity. Such individuals have idiopathic type 1 diabetes. Although the incidence of new-onset type 1 diabetes in older adults is very low, effective treatment of type 1 diabetes may prevent or delay the development of long-term complications and increased mortality. Thus, people who develop type 1 diabetes earlier in life may live to old age and therefore become a part of the spectrum of diabetes mellitus in an older adult population.
Approximately 90% of older adults with diabetes have type 2 diabetes mellitus. Hyperglycemia, often asymptomatic, is the hallmark and DKA is not part of the clinical syndrome. Obesity is often present, and resistance to insulin’s metabolic effects is a characteristic feature. Impaired insulin secretion is also part of the picture, but severe absolute insulin deficiency is not. While there is a strong genetic predisposition to development of type 2 diabetes, its etiology remains unknown and is likely to be both highly heterogeneous and multifactorial. The interaction between genetics, lifestyle factors, and aging in the development of type 2 diabetes is discussed later in this chapter in the section on “Pathophysiology of Diabetes and Its Complications.”
The 1997 ADA classification for diabetes mellitus identifies a number of specific conditions that lead to development of diabetes mellitus, each of which is relatively uncommon. One group of disorders includes genetic defects of the pancreatic beta cell. The clinical phenotype for these genetic disorders is maturity-onset diabetes of youth (MODY). These genetic disorders have autosomal-dominant inheritance with first presentation of asymptomatic hyperglycemia early in life. Patients with MODY can live to old age, however, and therefore can be a part of the spectrum of diabetes in an elderly population. The metabolic disorder in some affected individuals maybe mild, whereas others may develop symptomatic hyperglycemia and long-term complications of diabetes mellitus similar to patients with typical type 2 diabetes. Another type of genetic defect has been identified in a few families in which insulin processing prior to secretion is impaired, which can predispose to development of hyperglycemia.
Other families have genetic defects affecting insulin action. Severe insulin resistance results, and when it is not adequately compensated for by increased insulin secretion, hyperglycemia occurs. Diseases of the exocrine pancreas can lead to damage to pancreatic beta cells, diminished insulin secretion, and hyperglycemia. Hyperglycemia also can occur in patients with excessive secretion of hormones that adversely affect carbohydrate metabolism, such as in acromegaly, Cushing’s syndrome, glucagonoma, and pheochromocytoma. Similarly, tumors making aldosterone can cause hyperglycemia as a result of hypokalemia-induced inhibition of insulin secretion. A number of drugs or toxins can impair insulin secretion or insulin action and lead to the development of hyperglycemia. Table 109-2 lists such drugs. Several genetic neuromuscular disorders are associated with diabetes mellitus, but they are rare in the older adult population.
Diuretics |
α-Adrenergic agonists |
β-Adrenergic blockers |
Alcohol |
Ca2+ channel-blockers |
Caffeine |
Clozapine |
Glucocorticoids |
Growth hormone |
Nicotine |
Nicotinic acid |
Nonsteroidal anti-inflammatory drugs |
Female sex steroids (estrogen/progesterone) |
Pentamidine |
Phenytoin |
Gestational diabetes mellitus (GDM) is a separate category in the 1997 ADA classification and refers to the first identification of an alteration in glucose metabolism during pregnancy. While GDM per se is not part of the spectrum of diabetes in older adults, a history of GDM maybe part of the background of an older woman presenting with type 2 diabetes. As GDM has been aggressively screened for and treated, increasing numbers of women reaching the geriatric age group will have this history, as GDM affects approximately 4% of all pregnancies in the United States. After pregnancy, 5% to 10% of women with gestational diabetes are found to have type 2 diabetes. Women who have had gestational diabetes have a 20% to 50% chance of developing diabetes in the next 5 to 10 years.
The rationale for the circulating glucose level criteria shown in Table 109-1 is based on prediction of risk for diabetes-related complications. Thus, a 2-hour value during the oral glucose tolerance test (OGTT) greater or equal to 200 mg/dL is a strong predictor of diabetes complications, even when the fasting glucose level is less than 126 mg/dL. The term “isolated postchallenge hyperglycemia” (IPH) is sometimes used for individuals who meet the 2-hour OGTT criterion for diabetes, but who do not meet the fasting glucose criterion.
There is no age adjustment in the recommended criteria for the diagnosis of diabetes mellitus because the same glucose level cut points that predict complications appear to apply regardless of age. There is no current criterion for diagnosis of diabetes mellitus based on measurement of hemoglobin A1c, as no level of hemoglobin A1c accurately identifies people who meet glucose level diagnostic criteria.
The 1997 and 2003 ADA criteria shown in Table 109-1 also identify intermediate categories of altered glucose metabolism that identify an earlier stage (prediabetes) in the development of diabetes mellitus. Impaired glucose tolerance (IGT) is based on results of an OGTT and the category of impaired fasting glucose (IFG) is defined from fasting glucose levels. Because OGTTs are not routinely performed in clinical practice, the IFG category allows easy identification of some, but not nearly all, of the individuals who would meet criteria for IGT if an OGTT were performed. People with IGT and IFG are at increased risk for cardiovascular disease, as well as for subsequent development of overt diabetes mellitus. There is no recommended age adjustment for the criteria for either IGT or IFG, as these criteria predict risk for subsequent diabetes and cardiovascular disease similarly in older people.
One advantage of the 1997 and 2003 ADA recommendations is that a simple fasting glucose level can be used to classify patients and establish a diagnosis of diabetes mellitus, or even the intermediate stage of IFG. Unfortunately, many older individuals who meet diabetes criteria by OGTT will be missed by this method (see “Epidemiology of Diabetes and its Complications” later in this chapter). The ADA recommends that screening to detect prediabetes or diabetes with either fasting glucose level or 2-hour OGTT be considered in all individuals older than age 45 years at 3-year intervals, particularly in those with a body mass index (BMI) ≥ 25 kg/m2. The rationale for such screening includes the high rate of undetected diabetes mellitus in population studies (discussed further subsequently in the section on “Epidemiology of Diabetes and its Complications”) and the substantial current evidence that early intervention delays the development of diabetes in people with prediabetes, including those older than 60 years of age. Yearly follow-up and retesting of people at higher risk should be considered. Such high-risk individuals include those who are overweight (BMI ≥ 25 kg/m2; see Chapter 39), those with a strong family history of diabetes that includes a first-degree relative, those with a history of prior GDM, people with hypertension or dyslipidemia (particularly elevated triglyceride and/or low high-density lipoprotein [HDL] cholesterol levels), and those who have had IFG or IGT on previous testing. In addition, members of ethnic groups with higher risks of diabetes should be considered for more frequent testing. Such populations include African-Americans, Hispanics, Pacific Islanders, Asian Americans, and Native Americans.
What about testing to classify the type of diabetes mellitus? The ADA reports do not define criteria for classifying types of diabetes mellitus. In theory, it is possible to measure insulin levels and various pancreatic islet antibodies to identify most individuals with type 1 diabetes. At this time, however, the differentiation between types of diabetes is primarily based on clinical history. While clinical history is imprecise, it is sufficient under most circumstances to guide treatment interventions. Insulin therapy should be a key part of the approach to patients with type 1 diabetes and interventions targeted at enhancing endogenous insulin secretion are unlikely to be useful for such individuals, even in the early stages of their disease. Insulin can also be used in patients with other types of diabetes depending on the clinical situation (as described in more detail subsequently in the section on “Insulin Therapy”). Accurate classification maybe more important in the future. For example, if interventions are identified to prevent progression of autoimmune diabetes, it would be important to establish a specific diagnosis early so that such interventions could be used appropriately.
Epidemiology of Diabetes and Its Complications
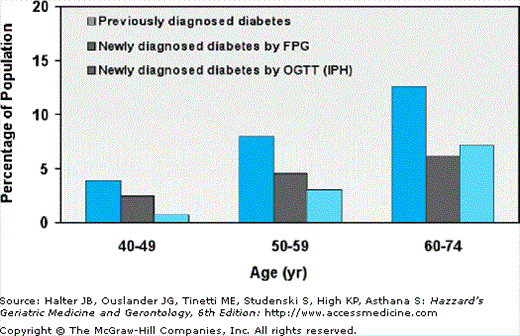
More than 50% of people in the United States with diabetes are older than 60 years of age. Figure 109-1 illustrates the dramatic age-related increase in the prevalence rate of diabetes mellitus in men and women in the United States. This analysis from the Third National Health and Nutrition Examination Survey (NHANES III) was carried out from 1988 to 1994 by the National Center for Health Statistics of the Centers for Disease Control on a nationally representative sample of the U.S. noninstitutionalized population. Based on the 1997 ADA criteria, approximately 25% of people older than age 60 years have diabetes mellitus. The high prevalence rate was confirmed in the population-based Cardiovascular Health Study and extended to people older than age 75 years. Approximately half of these older affected persons had a physician diagnosis of diabetes mellitus, but the other half were previously undiagnosed. Among the 12% to 13% of previously undiagnosed older people, approximately half were detected by an elevated fasting glucose. However, the other half had IPH, requiring an OGTT to detect the presence of diabetes.
Figure 109-1.
Prevalence of type 2 diabetes among elderly people according to age and ADA diagnostic criteria (National Health and Nutrition Examination Survey [NHANES] III). IPH, isolated postchallenge hyperglycemia. (Data from Harris MI, et al. Prevalence of diabetes, impaired fasting glucose and impaired glucose tolerance in US adults. Diabetes Care. 1998;21:518, and Resnick HE, et al. American Diabetes Association diabetes diagnostic criteria, advancing age, and cardiovascular disease risk profiles: Results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2000;23:176.)
Comparison with similar data collected in the late 1970s during NHANES II indicates that the prevalence of diabetes increased by 30% to 40% during the interval between studies. Diabetes prevalence varies by ethnic group, with the lowest rates in non-Hispanic whites, higher rates in non-Hispanic blacks, and the highest rates in Mexican Americans. Even higher rates are observed in some Native American populations. These ethnic group differences appear to persist throughout the age range.
The prevalence, or percent of the population with diabetes mellitus at any given age, includes both incident or new cases plus all of those with diabetes who were diagnosed at an earlier age. Part of the dramatic age-related increase in prevalence of diabetes is a result of survival of people initially diagnosed at middle age. However, other studies of the incidence of diabetes indicate an age-related increase and the prevalence of undiagnosed diabetes in NHANES III and other studies also increases with age.
After excluding people who meet criteria for diabetes mellitus, the prevalence rate for IGT and IFG also both increase with age. In NHANES III, approximately 14% of the U.S. population older than 60 years of age had IFG and approximately 20% met criteria for IGT, rates substantially higher than for younger individuals. When prevalence rates for both diabetes and IGT are combined, the rate in elderly people in the United States exceeds 40%. It could be argued that a finding prevalent in nearly half of the population should not be considered “abnormal” and that the criteria for these conditions should thus be age-adjusted. However, these criteria identify older people at risk for progression to overt hyperglycemia and at increased risk for the complications of diabetes.
Older adults with diabetes mellitus are susceptible to all the usual complications of diabetes. Although clinical outcomes of many of the diabetes complications including end-stage renal disease, loss of vision, myocardial infarction, stroke, peripheral vascular disease, and peripheral neuropathy all increase with age in the absence of diabetes, their incidence and cooccurrence are all exaggerated by the presence of diabetes, as summarized in Table 109-3. Diabetes-specific pathologic findings exist for some of these complications, but pathological information is generally not available for epidemiologic studies that depend on the more gross clinical outcomes. It is more difficult to be certain of the specific role of diabetes, and especially of hyperglycemia per se, in contributing to an outcome such as visual loss or end-stage renal disease in an older patient, as compared with a younger patient with diabetes, who would not otherwise be expected to have one of these problems.
COMPLICATION | RELATIVE RISK* |
|---|---|
Macrovascular disease | |
Coronary heart disease | 2 |
Stroke | 2 |
Amputation | 10 |
Microvascular disease | |
Blindness | 1.4 |
Renal disease | 2 |
Neuropathy | Uncertain |
In an older adult population, the presence of hyperglycemia and diabetes mellitus should be viewed as a risk factor for such complications, analogous to hypertension or hypercholesterolemia. Using this approach, there is substantial evidence that the presence of diabetes in an older adult increases risk for adverse outcomes, as summarized in Table 109-3. Overall, it is estimated that a diagnosis of diabetes is associated on average with a 10-year reduction in life expectancy. However, this figure becomes progressively reduced at advanced old age when risks of competing causes of mortality rise exponentially. Nonetheless, diabetes is associated with a higher mortality rate at any age, approaching twice the rate in older people of comparable age without diabetes in some studies. Similarly, the rates of myocardial infarction, stroke, and end-stage renal disease are increased approximately twofold, and the risk of visual loss is increased approximately 40% in older people with diabetes. This level of increased relative risk may appear modest on an individual level. However, since the elderly population has by far the highest rates of these conditions, the increase in absolute risk is more substantial. Thus a twofold relative risk increase represents a very large number of added adverse outcomes. The risk for lower-extremity amputation is dramatically increased in older people with diabetes mellitus, approximately 10-fold greater than that for older people without diabetes.
Pathophysiology of Diabetes and Its Complications
Although the rate of new onset of type 1 diabetes is relatively low in older people, the mechanism for type 1 diabetes does not appear to be different in this population. Usually, patients with type 1 diabetes have markers of immune destruction of pancreatic beta cells such as islet cell antibodies, antibodies to insulin, or other pancreatic beta cell-specific antibodies. There are also strong human leukocyte antigen (HLA) associations.
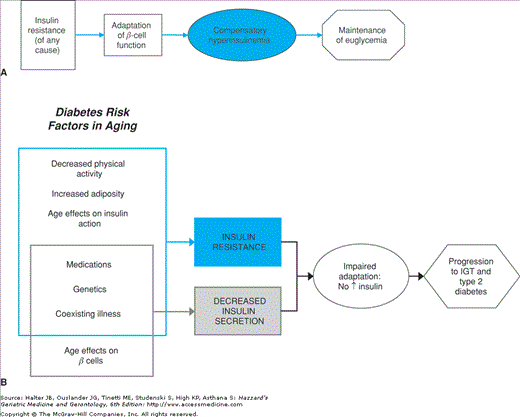
Type 2 diabetes is by far the most prevalent form in older adults. Autoimmune destruction of pancreatic beta cells is not observed. Limited pathologic investigation suggests that total beta cell mass maybe moderately reduced, but severe loss of beta cell mass is uncommon. The pathophysiology of type 2 diabetes is unknown, but it appears to occur as a result of a complex interaction among genetic, lifestyle, and aging influences, as illustrated in Figure 109-2. The heterogeneity of type 2 diabetes likely reflects the varying contributions of each of multiple factors to the development of hyperglycemia in a given individual or family.
Figure 109-2.
Model for age-related hyperglycemia. (A) Normal adaptation to insulin resistance. In normal individuals, pancreatic beta cell function compensates for insulin resistance with an adaptive response resulting in hyperinsulinemia, but maintenance of normal glucose levels (euglycemia). (B) Multiple risk factors for type 2 diabetes associated with aging predispose older adults to develop hyperglycemia. Some of these factors contribute to increased insulin resistance and some to decreased insulin secretion. As older individuals with impaired pancreatic beta cell function are unable to adapt adequately to insulin resistance, impaired glucose tolerance (IGT) and progression to type 2 diabetes can occur. A vicious cycle may ensue as hyperglycemia develops and leads to further deterioration of beta cell function and more severe insulin resistance. (Adapted from Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284:E7.)
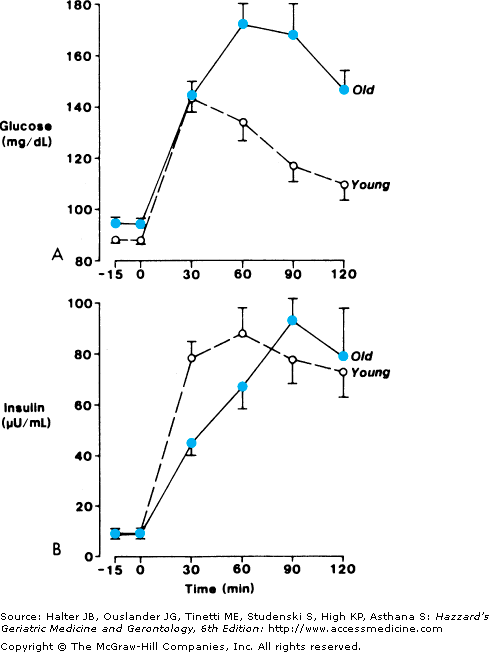
Many studies have demonstrated age-related glucose intolerance in humans, as illustrated in Figure 109-3. In normal people who do not meet criteria for either diabetes mellitus or IGT, there is a slight age-related increase in fasting glucose levels and a more dramatic slowing of return to normal of glucose levels following an oral glucose challenge.
Figure 109-3.
Plasma glucose (upper) and insulin (lower) levels before and following oral ingestion of 100 g of glucose in healthy old (n = 18) and young (n = 18) subjects matched for relative body weight and socioeconomic group. Subjects were eating an ad libitum diet. Note the slight elevation of fasting glucose levels in the old group and the delay in recovery of glucose levels following oral glucose. Also note the overall similarity of insulin levels between old and young subjects. (Reprinted with permission from Chen M, et al. The role of dietary carbohydrate in the decrease glucose tolerance of the elderly. J Am Geriatr Soc. 1987;35:417.)
There is a decline in sensitivity to the metabolic effects of insulin with age. An age-related impairment of intracellular insulin signaling reduces insulin-mediated mobilization of glucose transporters, which are critical to glucose uptake and metabolism in insulin-dependent tissues such as muscle and fat. There is currently little evidence for an age-related impairment of insulin effects on protein or fat metabolism.
An absolute or relative increase of body adiposity, particularly central body adiposity, has been well-documented with advancing age and appears to account in large part for the age-related increase in insulin resistance. Decreased physical activity is associated with insulin resistance and exercise training can improve insulin sensitivity. Thus, diminished physical activity in an older individual can also contribute to decreased insulin sensitivity. Both in animal studies and in humans, it has been difficult to demonstrate a residual effect of aging on insulin action when the changes in body composition and physical activity are controlled for.
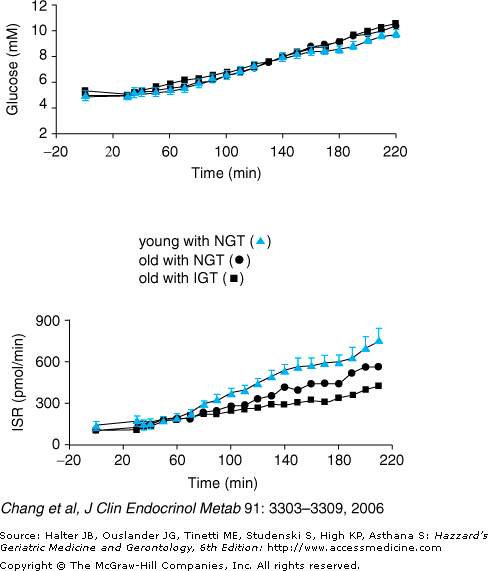
The progression from normal glucose tolerance to IGT and type 2 diabetes in aging is characterized by progressive defects in pancreatic beta cell function. Animal studies have demonstrated an age-related decline in beta cell function. However, there has been great variability in previous studies examining insulin secretion in older people. These human studies, however, may have failed to address adequately the importance of the normal adaptive response to insulin resistance. As most older individuals demonstrate insulin resistance, as already described, a compensatory increase of insulin secretion, leading to increased circulating insulin levels would be expected. As illustrated in Figure 109-2A, this is the normal adaptive response to insulin resistance that is observed in younger people who have obesity or other causes of insulin resistance. Such individuals have elevated fasting insulin levels and an increased insulin response to a glucose challenge. However, both basal and stimulated insulin levels in older adults are similar to those of insulin-sensitive young people, as illustrated in Figure 109-3, despite the coexistence of insulin resistance in the elderly subjects. Furthermore, when older and younger people are carefully matched for degree of insulin sensitivity, absolute impairments in betacell function with normal human aging have been demonstrated. Such defects are greater in older people with IGT, as shown in Figure 109-4. Impaired pancreatic beta cell adaptation to insulin resistance is an important contributing factor to age-related glucose intolerance and risk for diabetes.
Figure 109-4.
Effect of age on insulin secretion rate (ISR) in humans with normal glucose tolerance (NGT) or impaired glucose tolerance (IGT) by American Diabetes Association criteria. Plasma glucose concentrations and ISR are shown over time during intravenous glucose infusions, comparing young with NGT (n = 15, mean age = 26 yrs), old with NGT (n = 16, mean age = 70 yrs), and old with IGT (n = 14, mean age = 70 yrs). Glucose levels during variable rate glucose infusion begun at time 0 were well-matched in the three study groups, and degree of insulin resistance was also similar in the three study groups. ISR was significantly and progressively decreased in the two older groups, with the greatest impairment in old IGT (P = 0.0002, old IGT vs. young and old IGT vs. old NGT; and old NGT vs. young NGT). Data are means ± SE. (Adapted from Chang AM, et al. Impaired β-cell function in human aging: response to nicotinic acid-induced insulin resistance. J Clin Endocrinol Metab. 2006;91:3303.)
As summarized in Figure 109-2, in the setting of impaired beta cell function, there is a maladaptive response leading to impaired insulin secretion and progression to IGT and type 2 diabetes. Hyperglycemia, in turn, is known to contribute directly to insulin resistance and to impair pancreatic beta cell function, thereby setting up a vicious cycle of maladaptive mechanisms.
Coexisting illness is another factor affecting both insulin sensitivity and insulin secretion in an older person. Both hypertension and hyperlipidemia are common in older people and have been associated with diminished insulin sensitivity. In fact, a metabolic syndrome has been described that includes coexisting hypertension, hyperlipidemia, central obesity, and glucose intolerance. Some have proposed that insulin resistance is a unifying feature linking the components of this metabolic syndrome; however, there is uncertainty about whether the insulin resistance in these circumstances is primary or a secondary result of these other conditions. Furthermore, any acute illness can precipitate hyperglycemia because of effects of stress hormones to cause insulin resistance combined with the α-adrenergic effects of catecholamines released during stressful illness to inhibit insulin secretion. Drugs that may be used by older people may also contribute to hyperglycemia by causing insulin resistance (see Table 109-2).
Finally, a pathogenetic sequence common to many syndromes in old age can be readily illustrated by diabetes in an elderly patient: Hyperglycemia becomes frank diabetes, leading to acute complications and such syndromes as hyperosmotic nonketotic coma or, with time, to microvascular or macrovascular complications such as renal disease, stroke, or myocardial infarction, with superimposed drug-induced aggravation of impaired glucose regulation and decreased physical activity, progressive disability, and functional decline; that is, a downward spiral of mutually reinforcing conditions perhaps triggered by an event such as influenza or a fall that would be readily withstood by a younger person, especially a younger person without diabetes.
There is a strong genetic predisposition to type 2 diabetes mellitus. For example, the concordance rate for type 2 diabetes in identical twins approaches 100%. However, the genetic predisposition is complex and likely a key part of the heterogeneity of this disorder. As described previously, there are a few simple genetic abnormalities that cause a diabetes syndrome such as MODY. However, even within this narrow subtype of diabetes mellitus, there is considerable heterogeneity, as different families have now been described with defects in at least six different genes, all resulting in a similar phenotype. Furthermore, there is heterogeneity within each gene defect, as various alterations of the gene have been described in different families.
The situation in typical type 2 diabetes is likely to be much more complex, with multiple genes involved in different individuals in different families. Ultimately, there may be hundreds of different subtypes of type 2 diabetes identified as different combinations of genetic alterations are identified in different families. As these genes are identified and their functions determined, new therapeutic approaches to treatment may develop, enabling targeting of therapeutic interventions to the specific genetically determined defects present. Genome-wide scanning of large populations has now identified at least 10 genetic markers associated with type 2 diabetes. However, these markers in aggregate account for only a small percentage of type 2 diabetes risk.
In summary, the high rate of diabetes in older adults likely represents the interaction of genetic background with the linked age and lifestyle-related changes in insulin secretion and insulin action described here.
Chronic exposure to hyperglycemia may contribute directly to the development of diabetes complications in a number of ways. One mechanism may be the interaction of glucose with proteins to cause protein glycosylation and subsequent formation of advanced glycosylation end (“AGE”) products. The AGE products can accumulate in proteins of slow turnover such as collagen, potentially leading to tissue damage and injury. Tissue exposure to high concentrations of glucose can also lead to accumulation of metabolic products of the aldose reductase system including nonmetabolized molecules such as sorbitol. Such accumulation can potentially affect cellular energy metabolism and contribute to cell injury and death. Given the complexity of the genetic background contributing to type 2 diabetes, the possibility also exists that some of the genetic background of an individual may directly contribute to the risk for one or more long-term complications of diabetes (e.g., end-stage renal failure) independently of the effects of hyperglycemia. However, such a possibility remains speculative. Interactions between diabetes and other comorbidities may contribute to the manifestation and severity of diabetes-related complications. Diabetic patients who also have hypertension are at greater risk for renal disease and for macrovascular disease than diabetic patients without hypertension. Similarly, neuropathy is more likely in a diabetic patient who is exposed to a neurotoxic agent.
Prevention
Type 2 diabetes is a gradually progressive disorder of carbohydrate metabolism that develops over a long period of time. It has been estimated that abnormalities of glucose regulation can be detected 8 to 10 years before the clinical diagnosis of type 2 diabetes is made. The importance of lifestyle factors in the pathophysiology of type 2 diabetes and the epidemiological associations of healthy lifestyles with reduced risk for diabetes suggest that lifestyle interventions might prevent the development of diabetes. Given the progressive nature of impairments of glucose regulation, there is great interest in trying to identify people early in this course who are at high risk and therefore candidates for intervention to prevent progression. There is now substantial evidence that high-risk individuals can be identified and that progression to type 2 diabetes can be delayed. People with IGT and obesity appear to benefit from such interventions. Individuals from high-risk ethnic groups or who have a history of GDM also appear to benefit.
In the largest of these studies, the multicenter Diabetes Prevention Program (DPP) in the United States, an effort was made to recruit older adults with IGT. A lifestyle intervention program including both caloric restriction and exercise was shown to be remarkably effective in reducing the rate of progression to type 2 diabetes, even though only 5% to 7% weight reduction was achieved. In the DPP, the lifestyle intervention program was even more effective in people older than age 60 years than in younger people at risk, reducing progression to diabetes by more than 70% as compared to a control group. Drug interventions have also been tested. Metformin was used in the DPP and was effective in slowing progression in younger adults, but had surprisingly much less effect in people older than age 60 years.
As more is learned about the genetics of type 2 diabetes, it may be possible to identify high-risk individuals early in life based on genotype and to effectively target interventions. The success of the initial intervention trials to prevent progression has raised the question, not yet resolved, about whether and how screening should be carried out to identify high-risk individuals for more widespread clinical interventions.
Presentation
The finding of an elevated glucose level on routine laboratory testing is the most common presentation for type 2 diabetes in an elderly person. The classical clinical hallmarks of diabetes are symptoms associated with marked hyperglycemia, including polydipsia, polyuria, polyphagia, and weight loss. Older patients with type 2 diabetes may present with such symptoms, although it is relatively uncommon for them to do so. Some of these patients may have sufficient hyperglycemia to cause mild classical symptoms, while others may have had gradual unexplained weight loss. Other older patients may present with atypical symptoms from hyperglycemia such as falls, urinary incontinence, fatigue, or confusion. Because type 2 diabetes may go undetected for years, some older adults first present with symptoms or findings related to diabetes complications, such as visual loss with classic retinopathy on examination, proteinuria, or symptomatic peripheral neuropathy.
With the profound insulin deficiency of type 1 diabetes, mobilization of fatty acids occurs leading to accelerated production of ketoacids, potentially resulting in life-threatening DKA. Although type 1 diabetes usually occurs in early life, it can occur at any age, including first presentation in an elderly patient. Development of DKA is the classic form of presentation for a patient with type 1 diabetes, but it is now recognized that people with type 1 diabetes may have long periods of abnormal circulating glucose levels before the development of DKA. Furthermore, with early detection of such hyperglycemia and appropriate diabetes management, an episode of DKA might never occur. An older person with type 1 diabetes may be particularly more likely to present with an indolent course.
Evaluation
Diabetes mellitus is a complex disorder that can have effects on many body systems, and its treatment may require a complex program including medication plus lifestyle changes. The choice of intervention strategies and the patient’s capability to adhere to a diabetes treatment program may be limited by the presence of other health problems, as well as by the patient’s living situation, economic status, availability of caregiver support, and the like. A comprehensive geriatric assessment (Chapter 11) is highly appropriate to provide the basis for developing a treatment plan for an older person with diabetes mellitus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree