Country
Income level
Population (2014)
Life expectancy at birth (years)
GNI per capita in 2014 (USD)
Age-adjusted prevalence (%)
Dutch speaking
Aruba
HI
103,400
75
24,990
13.6
Curacao
HI
155,900
77
–
14.5
Sint Maarten
HI
37,660
76
–
14.2
Suriname
UMI
538,200
71
9470
11.1
English speaking
Anguilla
–
14,100
–
–
12.6
Antigua and Barbuda
HI
90,900
76
13,360
13.3
Bahamas
HI
383,100
75
20,980
14.2
Barbados
HI
283,400
75
14,960
12.4
Belize
UMI
351,700
74
4350
15.9
Bermuda
HI
65,180
81
106,140
12.8
British Virgin Islands
–
28,100
–
–
12.6
Cayman Islands
HI
59,170
–
–
14.3
Dominica
UMI
72,340
77
7070
10.9
Grenada
UMI
106,300
73
7850
9.4
Guyana
LMI
763,900
66
4170
15.9
Jamaica
UMI
2.721 million
73
5220
10.4
Montserrat
St Kitts and Nevis
HI
54,940
71
14,490
13.0
St Lucia
UMI
183,600
75
7080
8.2
St Vincent and Grenadines
UMI
109,400
73
6560
10.0
Trinidad and Tobago
HI
1.354 million
70
15,550
13.0
Turks and Caicos Islands
HI
33,740
–
–
–
US Virgin Islands
HI
104,200
80
13,660
12.1
French speaking
Haiti
LI
10.57 million
63
820
6.7
Martinique
HI
386,500
–
–
14.3
Guadeloupe
HI
403,750
79
–
6.3
St Martin
HI
31,530
79
–
–
Spanish speaking
Cuba
UMI
11.38 million
79
5880
8.1
Dominican Republic
UMI
10.41 million
73
6030
11.4
Puerto Rico
HI
3.548 million
79
19,310
13.0
The total regional population was 41.9 million in 2014. According to the World Bank, the median per capita income was $8995 in 2014 (http://data.worldbank.org/region/LAC), but the range is wide as countries vary from low to high income (Table 8.1). Tourism is a major source of income for many Caribbean nations as the region has a high literacy rate, tropical weather and relative political stability.
Life expectancy has been increasing over several decades. It increased by 5 years between 1990 and 2013 [2], and it is now 74 years for men and 77 years for women according to the Pan American Health Organisation (PAHO) (http://www.paho.org/hq/index). Hence, the Caribbean is rapidly ageing region. Simultaneously, infant and child mortality has fallen.
Coupled with these demographic changes, the Caribbean is undergoing a rapid nutritional and epidemiologic transition like many regions of the world [3]. In the 1940s, the most common causes of morbidity and mortality were infections. However, over the past 40 years, communicable diseases account for less than 10 % of the total mortality. Atherosclerotic complications, i.e. coronary artery disease and stroke, are now the leading cause of mortality (accounting for ~7 out of 10 deaths), and diabetes is the third reported cause in several countries. At the same time, several Caribbean countries have a double burden of infectious diseases (especially HIV/AIDS and newly emerging diseases such as chikungunya) and non-communicable chronic diseases (NCDs), which are assuming epidemic proportions contributing to morbidity and mortality [4].
Classification and Unique Aspects of the Pathophysiology of Type 1 and Type 2 Diabetes in the Region
Type 1A
Exposure to human enteroviruses has been implicated as an environmental factor that could trigger and accelerate the autoimmunity leading to type 1A diabetes. In Cuban studies, the presence of enterovirus RNA in sera from newly diagnosed patients with type 1 diabetes was significantly higher than in healthy controls (27 % vs. 3 %) [5, 6]. Also, enterovirus RNA was detected in sera of first-degree relatives of islet cell antibody-positive patients compared to healthy controls (16 % vs. 0 %). Molecular mimicry or the hygiene hypothesis are plausible explanations for these phenomena similar to other populations and regions.
The human T lymphotropic virus type 1 (HTLV-I) virus is endemic in the Caribbean with a prevalence of 2–5 % [7]. While endocrine disorders such as hypercalcaemia have been reported with HTLV-I, there is relatively little data about hyperglycaemia. One study [8] found a higher than expected seroprevalence of HTLV-I in Jamaicans with type 1 diabetes (i.e. 17 %). However, this initial finding is insufficient to prove a causal association and needs further study. Interestingly, HTLV-I virions use the glucose transporter type 1 (GLUT1) to infect CD4(+) lymphocytes [9].
Type 2 Diabetes
Role of Lifestyle Factors
Obesity is rising in the Caribbean as part of the global pandemic. The evidence for its role as a pathophysiologic driver of incident diabetes is overwhelming, and this is true also for the Caribbean. The population-attributable risk of body mass index for incident diabetes in the Caribbean is 66 %, and it is 80 % for waist/hip ratio, highlighting the role of adiposity [10]. The International Collaborative Study on Hypertension in Blacks (ICSHIB), which was an ecological study on the burden of NCDs in the African Diaspora, showed that the prevalence of obesity increased progressively from West Africa to the Caribbean and then to North America [11–13]. This geographical gradient in obesity also parallels the gradient in the per capita gross national product supporting a nutritional transition as the cause. Also worrisome is that the rates of annual weight increase in Jamaicans (1.37 kg/year) are significantly greater than African Americans (0.52 kg/year) and Nigerians (0.31 kg/year) [14]. Presumably this steep rise in weight gain is due to the effects of rapid cultural changes in a transitional society, and it does not bode well for incident diabetes rates.
Caribbean women have a disproportionate burden of obesity, as approximately one third of Caribbean women were obese and another third were overweight, and these rates are increasing in many countries, such as Barbados and Dominica [11]. Obesity is about half as common in men. This sexual dimorphism for obesity is different to other regions of the world where there is parity, or even greater risk, among men.
The prevalence of diabetes is closely correlated to the BMI and the amount of intra-abdominal fat (as measured by the waist circumference) across the east-to-west gradient of the Diaspora (Figs. 8.1 and 8.2) [15]. The trends in diabetes incidence and mortality paralleled secular changes in obesity. As evidence, obesity rates in Cuba plummeted in the early 1990s driven by an economic crisis, but there was a rebound followed by an overshoot several years later to its current prevalence of ~53 % when the economy and nutrition improved [16]. The population-wide increase in weight was immediately followed by a 116 % increase in diabetes prevalence and 140 % increase in diabetes incidence. A 49 % rise in diabetes mortality followed 6 years later. However, a notable exception is that Indo-Trinidadian men do not have strong correlation of diabetes prevalence with BMI [17], although this may reflect differences in intra-abdominal adipose tissue deposits.
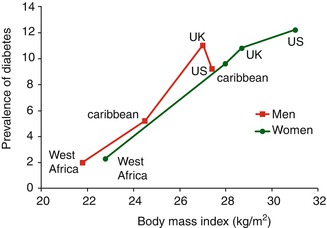
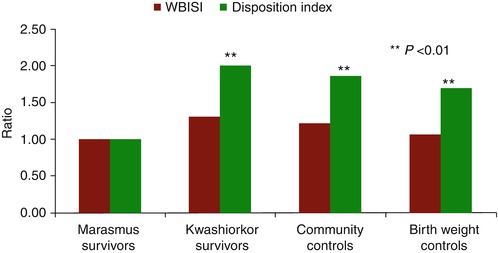

Fig. 8.1
Prevalence of diabetes mellitus (%) in the African Diaspora according to body mass index (Redrawn from data in Ref. [15])

Fig. 8.2
Ratios after age, sex and BMI adjustment of glucose metabolism (WBISI and disposition index) of kwashiorkor survivors, community controls and birth weight controls compared to those of marasmus survivors. Notes: (1) ratios represent the relative differences in each outcome between the kwashiorkor group and controls with the marasmus (comparator) group. (2) Kwashiorkor is a severe acute malnutrition that occurs with moderate wasting (<60 % weight-for-age) and nutritional oedema. (3) Marasmus is a severe acute malnutrition occurring with severe wasting (<60 % weight-for-age) and no oedema. (4) WBISI is the whole-body insulin sensitivity index. (5) Disposition index is derived from oral glucose tolerance testing Data are from Ref. [44]
There is a sexual dimorphism in diabetes prevalence as women have higher rates [18]. Although much of the variance is due to the higher prevalence of obesity in women, there is significant residual confounding by other factors, and this area needs more study. Clinical prediction models for diabetes in the Caribbean rely on the predictive ability of obesity using cut points of BMI (≥30 kg/m2) or central obesity (waist circumference >94 cm in men and 80 cm in women) [19, 20]. However, waist circumference is not superior to BMI in Jamaicans in its ability to predict incident diabetes [21]. Other data however suggest that the BMI cut-offs should be lower, i.e. 24.8 kg/m2 (men) and 29.3 kg/m2 (women). For waist circumference, these would be 88 cm and 84.5 cm for men and women, respectively [21].
Weight gain in Afro-Caribbean people may also be modified by the distribution of fat. Namely, ectopic fat deposits in the liver and within the fascia surrounding the skeletal muscle (i.e. intermuscular adipose tissue or IMAT) may be independent risk factors for insulin resistance and diabetes. Afro-Caribbean women have large depots of subcutaneous fat and better adiponectin levels than men [22], so the roles of total fat, central adiposity and fat topology remain to be delineated to see if they would explain some of the excess risk of diabetes in Caribbean women. There is a dearth of data about hepatic steatosis in the region. African Americans have greater IMAT compared to Caucasians even after matching for the total body fat and skeletal muscle mass [23]. Afro-Tobagonian men have high amounts of intramuscular fat infiltration as measured by peripheral quantitative CT scan. The degree of IMAT is positively correlated with glycaemia, and this may also be modified by a family history of diabetes. Much of the variability in IMAT may be genetic, as the residual heritability (due to additive genetic effects) was 35 % [24]. IMAT may thus be of equal or greater importance than central adiposity [25]. The ectopic deposition of lipids may be higher in obese Afro-Caribbean women than in obese white women since African-American women have higher rate of fatty acid uptake and higher expression of fatty acid-transporting proteins [26].
Physical activity seems to be declining while sedentarism is increasing as part of the epidemiologic transition. The levels of physical activity in Barbadian young people are comparable to American youth [27], and both are low. There is also sexual dimorphism in physical activity as Caribbean women generally report lower levels of activity. The importance of this is underscored in urban Jamaica where severe or energetic physical activity was uncommon in men, but non-existent in women [28]. The more recent ecological study, Modelling the Epidemiologic Transition (METS), using accelerometry confirmed that moderate-to-vigorous activity only occurred for 12 min daily in Caribbean women and was lower than African populations, but similar to American women [29]. While men had moderate-to-vigorous activity about 10 min more than the women [30], they were still relatively inactive although they remained lean. The protective effect of physical activity for incident diabetes is similar to other populations in a cross-sectional analysis. So, after adjusting for body composition, a one-unit increase in physical activity level (i.e. expending 590–670 kJ or about 20 min of brisk walking daily) was associated with a 20-fold reduction in the risk of diabetes [28].
Role of Developmental Factors
It is well established that low birth weight is associated with the development of NCDs in later life [31, 32]. Birth weight is a crude marker or summation of intrauterine growth, which in turn is determined by genetic factors, maternal body composition, maternal nutrition and placental sufficiency. So, children with low birth weight are more likely to have experienced growth restraint due to intrauterine nutritional restriction or much less commonly, a genetic predisposition to low birth weight [33]. However, the association of birth weight with type 2 diabetes is “J” or “U” shaped, i.e. the prevalence of diabetes is increased in individuals at both extremes of birth weight. The mechanisms underlying this relationship are not clear. However, both beta-cell dysfunction [34, 35] and insulin resistance [34–36] in childhood and adulthood may occur at the extremes of birth weight. Other pathophysiological mechanisms involved in low-birth-weight individuals include hypothalamic-pituitary-adrenal axis activation, visceral adiposity, changes in adipocytokines and altered appetite. Large-for-gestational-age children are more likely to be the offspring of glucose-intolerant mothers. Thus, they experience intrauterine hyperglycaemia causing fuel-mediated teratogenesis, which per se may induce insulin resistance and type 2 diabetes in later life. This was demonstrated in the international Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study which included more than 1200 Afro-Barbadian mothers [37].
Interestingly, fasting glucose was inversely related to birth weight in peri-pubertal Jamaican boys, but directly associated with girls [38]. This sexual dimorphism might be due to girls being intrinsically more insulin resistant as argued by some [39]. However, girls also have an earlier onset of puberty (an insulin-resistant state). Earlier menarche and greater breast development in Jamaican children were associated with higher fasting glucose even after adjusting for current BMI or prior growth rates [40]. In fact, fasting glucose increased by 0.6 mmol/L for each year reduction in the age of menarche.
In Jamaican children, shortness at birth and increased current weight are independent predictors of insulin resistance, as measured by 2-h insulin levels [41] and increased glycosylated haemoglobin levels [42]. However, data from a longitudinal birth cohort showed no relationship of birth size with insulin resistance measured in childhood [38] or in youth [43]. Probably, postnatal growth plays a role in the development of insulin resistance. So, children with faster postnatal growth during childhood (i.e. from ages 2 to 8 years) had greater insulin resistance, as measured by HOMA, in later life [38].
Prenatal factors may play a role in beta-cell dysfunction in later life as seen in animal models. So, greater maternal weight gain in pregnancy and smaller birth size are associated with reduced beta-cell function in youth [43]. This phenomenon is more clearly seen if severe acute malnutrition in infancy also occurs. Namely, if children with low birth weight are weaned early onto low-calorie feeds, they are more likely to develop marasmus (typified by severe wasting, i.e. weight-for-age <60 % but without nutritional oedema). Adult survivors of marasmus have more impaired glucose intolerance (odds ratio 10.9) than age, sex and BMI-matched community controls [44]. They have marked beta-cell dysfunction as measured by an oral disposition index, but were only marginally more insulin resistant.
It is therefore possible that intrauterine growth restriction and/or postnatal undernutrition may impair the development of beta cells during this plastic period of islet development during infancy leading to epigenetic changes that decrease insulin and PDX-1 gene expression. This would result in reduced beta-cell mass and glucose-stimulated insulin secretion in later life. Historically, up to 5 % of Caribbean populations experienced childhood severe acute malnutrition up to the 1950s, and some of these individuals may contribute to the burden of diabetes today. In Jamaican adults, declining beta-cell function is more of a driver of incident glucose tolerance more than worsening insulin resistance [45].
Other developmentally influenced mechanisms can also be in play. Hypothalamic-pituitary-adrenal axis activation by early life events could account for some of the association with glycaemia. Hence, lower birth size and earlier gestational age are associated with higher nocturnal cortisol, which in turn is associated with lower glucose effectiveness in adulthood [46]. Chronic inflammation of adipose tissue has also been implicated. As an example, faster growth in the first 6 months of life is associated with higher serum adiponectin levels in later life [47]. This implies that growth faltering in early infancy may lead to hypoadiponectinaemia in later life, which in turn is associated with insulin resistance and glucose intolerance. Finally, there may be an interaction with socio-economic status, i.e. young adults whose mothers had lower socio-economic status during pregnancy have more adverse outcomes associated with low birth size compared to those from higher status [48]. This may imply the action of other unknown environmental factors and possibly even endocrine disruptors.
The implication of these observations is important for Caribbean people. At present the average birth weight is ~3.1 kg, but there are a significant number of low-birth-weight and macrosomic babies, as well as obese mothers. If these children have growth faltering in early infancy (the first 6 months of life), or rapid growth in late infancy/childhood, they are at an increased risk of type 2 diabetes. Also, when this second generation of women later conceive, their offspring may be exposed to a hyperglycaemic intrauterine environment (and thus be born macrosomic) or experience placental insufficiency (and thus be born small for gestational age). Both conditions would increase the risk of glucose intolerance in the third generation.
Role of Other Metabolic Factors
Other metabolic factors may be involved in the development of type 2 diabetes, such as adipocytokines and oxidative stress. In Caribbean people, hypoadiponectinaemia is associated with incident glucose intolerance (OR ~0.93) [49, 50]. Heritability estimates of adiponectin suggest that genetic factors also influence the interindividual variation in circulating adiponectin levels and therefore the risk of glucose intolerance [51].
While oxidative stress (e.g. lipid peroxides such as isoprostanes) [52] and inflammatory markers (e.g. sialic acid and highly sensitive C-reactive protein) [53] may be involved in diabetic complications in Afro-Caribbean persons, they may not be involved in the pathogenesis of type 2 diabetes [45], but these studies may be underpowered. Similarly, glutathione levels are marginally lower in patients with type 2 diabetes, but are significantly low in diabetic persons with microvascular complications [54].
Chronobiology may play a role in a sex-specific manner. In Caribbean men, insufficient sleep (i.e. <6 h) or excessive sleep (>10 h) was associated with diabetes when adjusted for age, BMI and family history of diabetes (OR of 2.7 and 4.4, respectively). Surprisingly though, in women sleeping less than 6 h was associated with a reduced likelihood of diabetes (OR 0.4) [55].
Atypical Ketosis-Prone Diabetes (AKPD)/Type 1B Diabetes
In 1955, Hugh-Jones published one of the first descriptions of diabetes in the Caribbean where he observed that type 2 diabetes was more common than type 1 diabetes in Jamaica [56]. He also described an unusual variant which he called “J-type” diabetes – “J” standing for Jamaica. In more recent times, there have been other acronyms for J- type such as atypical diabetes, phasic insulin-dependent diabetes, ketosis-prone diabetes, type 3 diabetes, ketosis-prone type 2 diabetes and Flatbush diabetes. This variant was associated with insulin resistance, phasic dependency of insulin and a lean phenotype. It most resembles type 1B diabetes in the WHO classification. It is more common in non-white populations, and marginal nutritional status may play a role in some persons. In the Caribbean, there seems to be fewer cases of AKPD conceivably because improved nutrition in the Caribbean over the past several decades is making undernutrition less common. At present, Caribbean clinics are swamped with classical type 2 cases.
Patients with AKPD have periods in which they are insulin-requiring resulting in ketosis, especially during metabolic stresses, e.g. infections [57]. At other times, their need for insulin decreases such that reasonable glycaemic control can be obtained with only lifestyle modification and/or oral antidiabetic agents. Some persons have insulin resistance and others have experienced malnutrition in childhood [58]. It is conceivable that they may have reduced beta-cell mass and glucose-stimulated insulin secretion due to malnutrition in early life similar to the marasmic child [44]. Their beta cells appear to be sensitive to catabolic conditions leading to transient, decreased glucose-stimulated insulin secretion. The precise metabolic triggers (e.g. glucotoxicity, adipocytokines, non-esterified fatty acids causing lipotoxicity) are not known [59].
APKD may be a heterogeneous collection of different phenotypes with different degrees of impaired beta-cell function and autoimmunity (i.e. anti-GAD65 and anti-IA-2 antibodies) [57]. In an African-American series of AKPD which also contained Afro-Caribbean patients, about half had no evidence of autoimmunity (A-) with preserved beta-cell function (β+), while 22 % were A-β-, 17 % were A + β- and 11 % were A + β + [60]. We do not have comparative data in Caribbean persons, but in one study of diabetes in Caribbean youth, who were not necessarily selected on the basis of having ketosis-prone diabetes, 30 % were A-β+, 41 % were A-β- and 39 % were A + β- or A + β + [59, 61]. As such, some persons who have less autoimmunity, i.e. antibody negative but with preserved beta-cell reserve, demonstrate a clinical course more in keeping with type 2 diabetes despite having periods of ketosis [62]. Persons with positive autoantibodies tend to eventually need insulin therapy, while persons with preserved beta-cell function may have periods of insulin independence [57].
A few candidate genes have been examined to explain this variant of diabetes, but no genome-wide association studies have been done to date. A missense mutation Gly574Ser in the transcription factor HNF-1α was thought to be a marker of AKPD in African-American children [63]. However, this candidate mutation was not significant in Afro-Caribbean patients [64]. Other investigators working with other ethnic groups found that variants in HNF-1α and HNF-4α are unlikely to be major contributors to the pathogenesis of type 1B diabetes [65], but this is controversial [66].
Maturity-Onset Diabetes of Youth (MODY)
Like most of the world, MODY is rarely seen in the Caribbean. Even though families demonstrating multigenerational inheritance of diabetes and other characteristics consistent with early-onset type 2 diabetes have been identified, evidence of autoimmunity or sequence variants in MODY-1 to MODY-6 genes were absent [67]. Insulin promoter factor-1 (IPF-1) mutations in familial early-onset diabetes mellitus in Trinidadians have been described [68].
Diagnosis of Diabetes and Prediabetes in the Region
Type 2 Diabetes
In the 1950s and 1960s, diabetes was uncommon in the Caribbean with rates <3 % [69–71]. For example, in 1958, the prevalence was 1.4 % after screening 2325 adults for glycosuria in Trinidad [72] and 0.73 % among 958 Jamaican adults [71]. Notably, women with diabetes outnumber men as far back as 1962 in Trinidad [69], even though surprisingly they were not more overweight than the men.
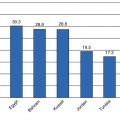
By the mid-1970s, PAHO sounded the alarm for a looming epidemic of diabetes and NCDs in the region [73]. Since then, the prevalence of diabetes has increased significantly, and the Caribbean, like many developing countries, has an epidemic of obesity and diabetes. The International Diabetes Federation (IDF) estimates that the age-adjusted prevalence in 2013 was 9.6 % for the Caribbean which is the second highest in the world after the Middle East and North Africa region. There is some variation in the rates even within countries, depending on the methods used to diagnose diabetes (fasting glucose vs. oral glucose tolerance testing, 1985 WHO diagnostic criteria vs. the 1997 criteria). Table 8.1 also gives a breakdown by country where logistic regression models were used to produce smoothed age-specific prevalence rates for adults aged 20–79 years, which can allow for country-to-country comparisons. Many countries have not carried out national surveys, and their prevalence rates are extrapolated from other Caribbean countries with a similar demographic profile. Clearly it is important for each country to have their data on the burden of disease.
Naturally, incidence rates of diabetes are also high, although there is some difference based on ethnicity. In Jamaica, which is predominantly of African ancestry, the rates are 15 and 20 per 100 person-years for men and women, respectively [21]. However in Trinidad, where approximately 35 % of the population are of Indian ancestry and another 35 % are of African, the incidence of diabetes is higher in Indo-Trinidadians. The rates of Indo-Trinidadian men were 24 per 1000 person-years compared to Afro-Trinidadian men (13 per 1000 person-years) [17]. This was also similar for Indo-Trinidadian women (23 per 1000 person-years) and Afro-Trinidadian women (14 per 1000 person-years) [17]. On account of the high incidence rates, the IDF has been predicted that the prevalence of diabetes in the Caribbean will increase by 37–60 % over the period by 2035. The epidemic is set to continue as there is a significant reservoir of prediabetes. Impaired fasting glucose occurs in ~3 % [74] and impaired glucose tolerance in 13.7 % [10]. Of note, most surveys did not perform oral glucose tolerance testing, so most of the region’s estimate by the IDF (13.2 %) is actually extrapolated from one late-1990s Jamaican survey [10]. Remarkably, there are little rural-urban differences [74] although there are differences by income status (lower income having higher prevalence). Undiagnosed diabetes is a heavy burden for the region and will increase the morbidity, mortality and loss of human capital. About 25 % of people with diabetes in the Caribbean are unaware of their status [74, 75].
Youth with Type 2 Diabetes
Although cases of type 2 diabetes have been reported in youth, prevalence data is missing in many countries. Anecdotally, these cases have been increasing with the increasing girth of the region’s youth. In the US Virgin Islands, the incidence of type 2 diabetes in youth age ≤19 years rose significantly between 2001 (5.3/100,000) and 2010 (12.5/100,000) giving an age-adjusted annual incidence rates of 9.6 per 100,000 [76]. In Trinidadian schoolchildren, urine screening had a positive predictive value of 65 % for detecting diabetes, and it showed a prevalence of 10·4/100,000 while 7·5/100,000 had IGT [77]. This would, of course, be an underestimate compared to blood screening. In a small Jamaican study, type 2 diabetes occurred in a third of cases of diabetes diagnosed in youth less than age 25 years [78]. Affected youth were more likely to be female, older at diagnosis, obese and have a higher blood pressure when compared to those with type 1 diabetes. However, the strongest predictor of type 2 diabetes was obesity measured by BMI [78].
Type 1 Diabetes
Like many regions, type 1 diabetes is uncommon in the Caribbean. Precise prevalence and incidence data are sparse in many countries, but data exist for Bahamas and the Eastern Caribbean. The prevalence was very high (31/100,000) in Bahamian youth <age 24 years with an incidence rate of 10.1/100,000 in those age 0–14 years [79]. Incident rates are 4–5 per 100,000 person-years in persons with African ancestry in the Eastern Caribbean [80, 81] rising to a peak in the US Virgin Islands (15.3 per 100,000 person-years) [76, 81] which may reflect their higher degree of genetic admixture. The rates show some seasonal variation and a tendency for a secular increase, which augurs for unknown environmental factors being part of the aetiology such as the accelerator hypothesis [80]. About half of patients with type 1 diabetes are type 1A (with anti-GAD65 and/or IA-2 antibodies positivity), and half are type 1B (i.e. autoantibody negative) [61].
Gestational Diabetes Mellitus (GDM)
The epidemiologic transition would be expected to increase the risk for gestational diabetes, as well as the number of women who enter pregnancy with type 2 diabetes. About 4–13 % of Jamaican women of child-bearing age have diabetes [10, 74]. There has been an apparent increase in the incidence of GDM in Trinidad. Preliminary data show that from 2005 to 2007, the prevalence of GDM increased from 1.7 to 6.7 % with a mean prevalence of 4.3 % [82]. Unknown genetic factors may play a role. A family history of early-onset autosomal-dominant type 2 diabetes seems to increase the risk of GDM in Jamaican women (12 % vs. 1.5 % in controls, i.e. OR 9.0) which would have implications for screening [83].
Women with prior GDM are at very high risk for incident type 2 diabetes. So, among Trinidadian women with prior gestational diabetes, 62 % develop diabetes and 17 % had IGT within 7 years [84]. These data suggest that 10–18 % of women convert to diabetes per year, and the conversion to IGT occurs in another 3–5 %/year. If confirmed, these rates are extraordinarily high compared to other regions of the world [84].
Genomic Landscape of Diabetes in the Region (Local Data, if Available, or Best Approximation)
With its unique history of migration and colonisation, it is no surprise that the Caribbean is an admixed region. Most persons claim African ancestry, and one study using 28 ancestry informative markers (AIMs) found that 84–90 % had West African ancestry, 10–12 % European ancestry and 0–3 % Native American ancestry [1]. Another study using 416 AIMs found Afro-Barbadians were ~77 % African, 16 % European and 7 % Asian [85]. However, Tobagonians had the lowest rate of admixture (<6 %) [86].
Spanish Caribbean populations may have more Native American ancestry [87], and this may play a role in the development of incident type 1 diabetes in these countries. Genetic susceptibility to type 1 diabetes is determined by a combination of HLA-DQ and DRB1 genes (or a gene in linkage disequilibrium with it). In a Cuban sample, a one-unit change in European admixture proportion was associated with a 5.7-fold risk for type 1 diabetes [88]. The HLA alleles DQA1*0501, *0301 DQB1*0201 and DRB1*0301, *0401 were susceptibility alleles, while DRB1*1501, DQA1*0102/3 and DQB1*0602 were protective. In Afro-Jamaican patients, DRB1*03-DQ2/DRB1*04-DQ8, DRB1*0401-DQ8 and DRB1*0408-DQ8 genotypes increase the risk of type 1 diabetes. The DRB1*1503-DQ6 and DRB1*03-DQA1*0401-DQB1*0402 haplotypes were protective alleles. This pattern in the Jamaicans is different from the protective and predisposing haplotypes on European populations [89]. Clearly more work is needed in this area.
For type 2 diabetes, there have been a few studies utilising a candidate-gene approach as well as genome-wide association studies, although some argue that genetic factors play only a minor role among Caribbean populations [90]. A family history of diabetes in any first-degree relative (parent, sibling) or in a grandparent is associated with a two- to fourfold increased risk of diabetes [10, 91]. A family history of diabetes is probably a summary statement for several interactive related genetic, but there is limited data on specific genetic factors involved in persons of Caribbean origin.
Among the candidate genes, the transcription factor, TCF7L2, which is involved in beta-cell dysfunction, was associated with glucose intolerance in 385 Afro-Caribbean persons living in the UK [92]. This seems persistent among nonmigrant populations as among ~1000 Jamaicans, TCF7L2 was associated with decreased beta-cell function (as measured by HOMA-%B) (unpublished data).
Mutations in the ATP-sensitive potassium channel (KCNJ11) in the beta cell are also associated with impaired glucose-stimulated insulin secretion. The common variant E23K was significantly associated with type 2 diabetes in Indo-Trinidadians (OR = 1.8) along with a few other (novel) missense mutations A94P and R369C and S118L (in an Afro-Trinidadian) [93].
Candidate-gene approaches for insulin resistance have not been very successful. The PC-1 (ENPP1) K121Q polymorphism is not significantly associated (Colin A. McKenzie, personal communication, 2015). Variants in peroxisome proliferator-activated receptor gamma (PPARG), and the obesity-associated gene, FTO, have not been well investigated in Caribbean populations. The Trp64Arg mutation of the beta3-adrenergic receptor has been associated with hyperglycaemia and obesity in women, but not in men [94].
Persons of African ancestry have more fat infiltration of skeletal muscle fat than Europeans. Since this is a heritable polygenic trait, a candidate-gene study examined non-synonymous coding variants in carnitine palmitoyltransferase-1B (CPT1B). CPT1B is an enzyme that regulates skeletal muscle mitochondrial beta oxidation of long-chain fatty acids. The G531L and I66V variants were associated with ectopic fat infiltration in the skeletal muscle among 1774 older men from the population-based Tobago Health Study [95].
Genome-wide association studies by the Genetic Investigation of ANthropometric Trait (GIANT) consortium showed several single nucleotide polymorphisms associated with adiposity. Using the phenotype, waist/hip ratio after adjusting for body mass index, they identified several SNPs (49 loci of which 33 were new), and the effect size was stronger in women among 20 of these SNPs. These SNPs localised to pathways involved in adipogenesis, angiogenesis, transcriptional regulation, white adipose tissue differentiation, insulin resistance, adipose inflammation (including adiponectin) and fat topography in the 14,371 individuals of non-European ancestry, which also included 2437 Afro-Jamaican individuals [96, 97].
Complications of Diabetes in the Region
Health Disparities in Complications
People of African ancestry including Afro-Caribbean populations have lower rates of myocardial infarction compared to Caucasians [98, 99], but conversely stroke [100] and renal failure [101] rates are higher. It is not clear why such disparities exist, but genetic factors [98], coexisting cardiometabolic risk factors [100], access to care and intensity of therapeutic control of risk factors are potential culprits. There is limited data on genetic factors influencing complications in diabetic Caribbean people. Persons of African ancestry have higher systolic blood pressures, but lower triglyceride and total cholesterol levels [99]. Hypertension occurs in 52 % and 35 % of diabetic Jamaican women and men, respectively [10], and there may be less nocturnal dipping of blood pressure [102]. Tobacco use is relatively low in diabetic persons [10] compared to developed countries. As a result, these co-morbid cardiovascular differences may explain some of the higher rates of stroke and renal failure (which are sensitive to blood pressure), and the less atherogenic lipid profile may reduce the risk of coronary artery disease. Notably, youth with diabetes have a more adverse cardiometabolic pattern and thus may be more at risk of complications [103], as was also seen in the SEARCH study.
Mortality and Macroangiopathy (Coronary Heart Disease and Stroke)
The leading cause of death in the Caribbean is ischemic heart disease, and stroke is the second of which diabetes is a major cause [104]. Also, diabetes is the leading causes of disability-adjusted life years. Mortality rates from diabetes have increased dramatically, i.e. 63 % from 1980 to 1990. Diabetes-attributable macrovascular complications are affected by ethnicity. The population-attributable mortality of Indo-Trinidadians is 2.9–6.9 times higher than other ethnic groups [98, 105], and most of this is due to diabetes-induced cardiovascular disease. The age-adjusted death rates due to diabetes in 2000 were 25, 46, 56, 58 and 108 per 100,000 world standard population for Suriname, Bahamas, Barbados, Jamaica and Trinidad, respectively, according to PAHO (Health Statistics for the Americas, 2006 Edition, PAHO). In North America, the rates are less than 16 per 100,000, so these data show significant health disparities. In Barbados, diabetes accounted for an excess mortality of 42 %, and there was a 9 % increase in all-cause mortality for each 1 % increase in A1c [91]. However, there is dearth in data in other Caribbean countries about specific macrovascular complications and their mortality. Cardiovascular disease is present in almost 60 % of hospitalised diabetic Jamaicans and is more frequent among women [106]. Silent MI may occur in a quarter of Guadeloupian patients especially if they have left ventricular hypertrophy [107]. The atrial natriuretic peptide rs5065 (2,238T>C) C allele seems to exert a protective effect (24 % vs. 41 %, OR 0.5) in a relatively small study in Guadeloupe [108].
Diabetes-related mortality is not limited to older age groups, as 38 % of deaths occurred in people under the age of 60 [109, 110]. Mortality in people with type 1 diabetes in the US Virgin Islands is high, as cumulative survival was 98 % at 10 years, but fell to 73 % at 20 years. This high incidence rate of T1D in the US Virgin Islands may be partially responsible for the high mortality rate also seen [111].
Diabetic Foot Disease and Amputations
Diabetic foot disease has major public health consequences for the region. Approximately one of every eight patients in diabetes clinics had a major foot complication (amputation, ulcers, infection). Factors associated with these complications were neuropathy (OR 9.3), high blood pressure (OR 7.9) and longer duration of diabetes (OR 1.32) [112]. Diabetic foot disease accounted for 30 % of admissions in Barbados and 89 % of diabetes-related admissions [113]. In fact, Trinidad uses 0.4 % of their gross domestic product solely to treat patients hospitalised for diabetic foot infections [114].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree