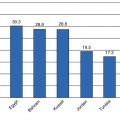
Race/ethnic group
Age-adjusted overweight prevalence (%)
Age-adjusted obesity prevalence (%)
Chinese
21.8
4.2
Filipino
33.0
14.1
Asian Indian
34.4
6.0
Japanese
25.9
8.7
Vietnamese
19.1
5.3
Korean
27.3
2.8
Other Asian and Native Hawaiian or other Pacific Islander
29.2
12.5
Glucose Metabolism and Insulin Resistance
Insulin resistance is a key contributor to diabetes risk in NHB, Mexican American, Asian American, and Native American populations. Compared to their NHW counterparts and independent of adiposity, NHBs, Mexican Americans, Asian Americans, and Native Americans have greater insulin resistance [7]. Studies in non-Mexican Hispanic Americans have yielded conflicting results. Glucose metabolic features differ among Hispanic Americans from different countries of origin. This may explain the higher prevalence of diabetes in Mexican Americans compared to those of Cuban American and South American descent. Asian Americans have lower beta-cell insulin secretion compared to NHWs [7]. The reduced beta-cell function in Asian Americans may also explain why they are at high risk for diabetes at lower levels of BMI [8].
Individual Racial/Ethnic Differences in Nonbiological Factors
Acculturation
Acculturation in Hispanic American immigrants results in an increase in dietary habits that promote obesity [9]. The association of acculturation with diabetes, however, is mixed and inconsistent. Even though acculturation appears to promote obesity, its association with diabetes may be offset by its positive influence on physical activity and access to care, in that screening and intervention occur sooner [9]. Japanese immigrants to the USA have a threefold higher rate of diabetes compared to their native Japanese counterparts [7].
Health Behaviors
Physical inactivity is an important risk factor for diabetes. NHBs, Native Americans, and Alaska Natives report less leisure-time physical activity than NHWs [10]. Mexican American women report less leisure-time physical activity than NHW and NHB women [10]. There are sparse data currently on physical activity in Asian American populations [4].
Smoking has also been identified as a risk factor for diabetes, independent of adiposity. Native Americans and Alaska Natives have a higher prevalence of smoking than NHWs [10]. NHBs and NHWs have similar smoking rates, while Mexican Americans have significantly lower smoking rates. In Asian Americans there is variation in rates of smoking, with the highest prevalence among Korean men and the lowest prevalence among Asian Indian men [4].
Genomic and Epigenetic Landscape of Diabetes
Results of several genome-wide association studies (GWAS) have linked the following common gene variants with a 15–20% increased risk of diabetes: reduced insulin secretion via reduce beta-cell mass (CDKAL1, CDKN2A, CDKN2B) and beta-cell dysfunction (MTNR1B, TCF7L2, KCNJ11) and increased insulin resistance related to obesity (FTO) and unrelated to obesity (IRS1, PPARG) [11]. While most of the early studies focused on individuals of European descent, several recent studies have demonstrated that these susceptibility loci are also present and associated with increased diabetes risk in ethnic minority populations. The limited amount of data in non-European ancestry populations does not suggest that the genetic architecture of diabetes differs across race/ethnic groups. Until recently, there were no GWAS analyses in non-European populations; however, several studies have identified novel diabetes-associated SNPs in specific race/ethnic groups that are being further evaluated (see Table 14.2).
Table 14.2
Novel GWAS-identified loci associated with type 2 diabetes in non-European populations [1]
Population | New identified SNP | Chromosome | Associated gene(s) | Associated physiological function |
|---|---|---|---|---|
South Asians [12] | Rs3923113 | 2 | GRB14 | Associated with insulin sensitivity |
Rs16861329 | 3 | ST6GAL1 | Associated with pancreatic beta-cell function | |
Rs1802295 | 10 | VPS26A | ||
Rs7178572 | 15 | HMC20A | ||
Rs2028299 | 15 | AP3S2 | ||
Rs4812829 | 20 | HNF4A | Associated with pancreatic beta-cell function | |
East Asians [13] | ||||
Loci with strong associations | Rs6815464 | 4 | MAEA | |
Rs7041847 | 9 | GLIS3 | Associated with pancreatic beta-cell development, insulin gene expression, and glucose | |
Rs6017317 | 20 | FITM2-R3HDML-HNF4A | ||
Rs6467136 | 7 | GCC1-PAX4 | ||
Rs831581 | 3 | PSMD6 | ||
Rs9470794 | 6 | ZFAND3 | ||
Rs3786897 | 19 | PEPD | ||
Rs1535500 | 6 | KCNK16 | May regulate glucose-independent insulin secretion in the pancreas | |
Loci with moderate associations | Rs16955379 | 16 | CMIP | |
Rs17797882 | 16 | WWOX | ||
NHBs [14] | ||||
Loci with strong associations | Rs7560163 | 2 | RBM43 | |
RND3 | ||||
Loci with nominal associations | Rs7542900 | SLC44A3 | ||
F3 | ||||
Rs4659485 | 2 | RYR2 | ||
MTR | ||||
Rs2722769 | 11 | GALNTL4 | ||
LOC729013 | ||||
Rs7107217 | 11 | TMEM45B | ||
BARX2 | ||||
Epigenetics represents the interface of environmental and biological factors and has implications in the predisposition of NHBs to developing diabetes. Low birth weight, fetal undernutrition, and maternal-fetal stress appear to be unique contributors to elevated diabetes and metabolic risk in NHBs. NHBs have lower birth weight than NHWs, which may be related to maternal conditions during pregnancy, including stressful life events, depressive and anxiety symptoms, economic inequality (e.g., lower income and education, less access to healthcare in NHBs), racial discrimination, residential segregation, neighborhood-level poverty, and maternal hypertension [15]. Smaller birth weight is linked to insulin resistance and diabetes, abdominal pattern of fat distribution, higher blood pressure, abnormal lipid profiles, and increased cardiovascular disease risk [15]. Studies have also linked low birth weight with elevated cortisol reactivity in childhood and adolescence [15]. And chronic cortisol exposure can contribute to abdominal adiposity and insulin resistance [16]. Maternal psychological stress and fetal overexposure to cortisol lead to the same metabolic abnormalities as fetal undernutrition [15].
Epigenetic changes in the pattern of cellular gene expression may influence how a fetus adapts to an adverse intrauterine environment which may increase survival in utero but predispose that individual to enhanced metabolic risk later in life [15]. Further studies are needed to determine how epigenetic changes contribute to the role of the fetal environment in racial/ethnic differences in future risk of obesity and diabetes [17].
Diagnosis of Diabetes and Prediabetes
In 2010 the American Diabetes Association (ADA) updated its clinical practice guidelines to include HbA1c criteria for the diagnosis of diabetes and prediabetes. In the context of using HbA1c as a diagnostic criteria in nondiabetic individuals, recent studies have suggested that non-glycemic factors may contribute to the higher HbA1c at similar levels of fasting glucose seen in NHBs, Hispanic Americans, Asian Americans, and Native Americans, compared to NHWs [18]. Suggested factors include variations in erythrocyte membrane permeability to glucose, regulation of glucose transport across the erythrocyte membrane, glycolytic rates, differences in nonenzymatic glycosylation reactions, and deglycation [18].
Two recent studies have examined the effect glycemia has on HbA1c differences in NHBs and NHWs with diabetes [19, 20]. One study used data from National Health and Nutrition Examination Survey III and the Screening for Impaired Glucose Tolerance Studies and found that NHBs with diabetes had significantly higher HbA1c than NHWs with diabetes, which persisted following adjustment for plasma glucose [19]. A subsequent study, using data from the Atherosclerosis Risk in Communities Study, however, came to a different conclusion [20]. This study attributed the higher HbA1c in NHBs with diabetes (compared with NHWs with diabetes) to higher fasting glucose and other covariates [20]. In addition, nontraditional glycemic markers, glycated albumin, and fructosamine, which are not subject to the effects of erythrocyte turnover and hemoglobin glycation, were also significantly elevated in NHBs with diabetes, compared to NHWs with diabetes [20]. Thus, these data support more hyperglycemia in NHBs with diabetes. As conflicting evidence is evolving about the contribution of non-glycemic factors to HbA1c levels in minorities with diabetes, caution should be used in applying HbA1c as the only measure to assess diagnosis of prediabetes and/or diabetes.
Diabetes Complications
Overall, ethnic minorities appear to be disproportionately affected by microvascular complications and mortality associated with diabetes, likely related to poorer glycemic and cardiovascular disease risk factor control; however, there are some notable paradoxes. NHBs with diabetes have a lower incidence of cardiovascular disease than NHWs with diabetes; however, once they have cardiovascular disease, they are more likely to die. NHBs have a higher rate of ESRD secondary to diabetic nephropathy but are less likely to die on dialysis than NHWs. NHBs, Native Americans and Alaska Natives, and Hispanic Americans are 2.3, 1.9, and 1.5 times more likely to die from diabetes than NHWs [21]. And while Asian Americans, overall, are 20 % less likely to die from diabetes than NHWs, there are variations among subgroups, such that Native Hawaiians and Filipinos living in Hawaii are 5.7 and 3.0 times more likely to die from diabetes than NHWs living in Hawaii [21].
Race/Ethnic Differences in Biological Factors Contributing to Complications
Glycemic Control
Several systematic reviews and meta-analyses have shown that ethnic minorities with diabetes have worse glycemic control than NHWs, which likely contributes to the higher risk of microvascular complications seen in these populations [22–24]. The proportion of diabetic ethnic minorities with poor glycemic control, defined as glycemia above a specific threshold, was significantly higher among NHBs, Hispanic Americans, Native Americans, and Asian Americans and Pacific Islanders [22]. Although discussed more in the context of using HbA1c as a diagnostic criteria for diabetes, recent studies have suggested that non-glycemic factors may contribute to the higher HbA1c at similar levels of fasting glucose seen in NHBs, Hispanic Americans, Asian Americans, and Native Americans, compared to NHWs [18]. However, HbA1c is similarly associated with prevalence and risk of microvascular and macrovascular complications, and mortality, among NHB and NHWs with diabetes [25–27], lending credence to true differences in glycemia between the two race/ethnic groups.
Cardiovascular Risk Factors
Hypertension is an important risk factor for diabetic nephropathy/end-stage renal disease (ESRD) and peripheral arterial disease and likely contributes to the higher prevalence of these complications in certain ethnic groups. NHBs have a higher prevalence of hypertension than NHWs [10]. Mexican American women have a higher prevalence of hypertension than NHW women; however, the prevalence of hypertension is similar among men in the two race/ethnic groups [10]. Native Americans and Alaska Natives have a lower prevalence of hypertension compared to NHWs and NHBs; however, there are regional differences [10]. Studies of blood pressure control and hypertension prevalence comparing NHWs to Native Americans and Asian Americans with diabetes are lacking. Research to date from clinical trials indicates that control of reversible risk factors, including hypertension, is equally effective in lowering the risk of nephropathy and cardiovascular disease in minority and NHW populations [28].
In a prior systematic review comparing low-density lipoprotein cholesterol between minority populations and NHWs with diabetes, Hispanic Americans had slightly lower low-density lipoprotein cholesterol than NHBs and NHWs. In this review, there were no studies comparing lipid control in NHWs with Native Americans and Asian Americans with diabetes [29]. While NHBs have lower triglycerides than NHWs [25], which may explain their lower risk of macrovascular disease, they generally have a higher prevalence of low high-density lipoprotein cholesterol, a strong cardiovascular risk factor [30].
Individual Race/Ethnic Differences in Nonbiological Factors (Health Behaviors) Contributing to Complications
Self-Monitoring of Blood Glucose (SMBG)
While some studies have shown no differences in SMBG by race/ethnicity, several have shown lower rates among NHBs, Hispanic Americans, and Asian Americans, compared to NHWs. Two studies found no difference in SMBG frequency in Native Americans compared to NHWs [31]. There are several barriers to SMBG, including inconvenience and intrusiveness, pain, lower socioeconomic position, education level, social class, and living in a high poverty area, many of which are disproportionately prevalent in ethnic minority groups [31].
Physical Activity and Smoking
As summarized above, ethnic minorities are less likely to engage in leisure-time physical activity, which can contribute to worse glycemic control and a propensity to developing microvascular complications [10].
Native Americans and Alaska Natives have a higher prevalence of smoking that NHWs [10], which can contribute to their higher risk of peripheral arterial disease and amputations. NHBs and NHWs have similar smoking rates, while Mexican Americans have significantly lower smoking rates. Therefore, smoking may not explain ethnic differences in peripheral arterial disease in these populations.
Coordination and Delivery of Diabetes Care Services: Health System Interventions Targeting Minority and Underserved Patients with Poorly Controlled Type 2 Diabetes
Evidence-Based General Diabetes Quality Improvement Interventions
Quality improvement strategies can target several areas—health systems (case management, team changes, electronic patient registry, facilitated relay of information to clinicians, continuous QI), healthcare providers (audit and feedback, clinician education, clinician reminders, financial incentives), or patients (patient education, promotion of self-management, reminder systems) [32]. Several prior meta-analyses have examined the impact of these intervention approaches on glycemic control and other metabolic control indices in patients with diabetes [32–34]. Shojania et al. performed a systematic review and meta-analysis of 58 studies of 66 distinct trials. These studies utilized a median of three quality improvement strategies and had a median follow-up of 13 months. The mean post-intervention HbA1c difference, compared to pre-intervention, was –0.42 % with greater reductions if baseline HbA1c was ≥8 % [33]. Strategies associated with at least a 0.5 % reduction in HbA1c after controlling for baseline HbA1c ≥8 % and study size included team changes (−0.67 %) and case management (−0.52 %). In comparative analyses, interventions that included case management reduced HbA1c significantly more than interventions that did not include case management, and of these types of interventions, the most effective case management interventions were those in which the interventionists could make independent medication changes [33]. This was confirmed in a subsequent meta-analysis of randomized controlled trials of disease management programs improving glycemic control in adults with type 1 and type 2 diabetes [34]. This same study suggested that interventions with high-frequency patient contact (several times/month) were more effective than those with low-frequency contact intervention [34].
Similarly, interventions that included team changes reduced HbA1c significantly more than interventions that did not include team changes, particularly those that included multidisciplinary, interactive teams [34]. Interventions with team changes remained significant after controlling for the presence of case management [33]. In those studies, adding a new team member alone was not effective, but rather, adding a team member with shared care between specialists and primary care providers or new team members with an expanded role were most effective.
A more recent meta-analysis expanded on Shojania’s prior study by including process outcome measures (proportion taking aspirin, statins, and antihypertensives and the proportion screened for retinopathy, foot abnormalities, and renal dysfunction) and additional non-glycemic outcome measures (LDL cholesterol, blood pressure, and proportion with controlled hypertension or quitting smoking) to evaluate the additional impact of multicomponent diabetes quality improvement interventions [32]. Overall, interventions resulted in lower HbA1c, LDL cholesterol, and blood pressure in those receiving compared to those not receiving the interventions [32]. These strategies also improved the likelihood that patients received aspirin therapy, antihypertensives, and screening for diabetic complications. Statin use, blood pressure control, and smoking cessation were unchanged. For patients with HbA1c ≥8 % intervention strategies that lower HbA1c ≥0.5 % included team changes, case management, patient education, and promotion of self-management; however, for patients with HbA1c < 8 %, facilitated relay was more effective in lowering HbA1c ≥ 0.5 % [32]. The only intervention strategy that was not effective in lowering HbA1c was clinician education [32]. These data suggest that greater improvements in HbA1c can be achieved utilizing QI intervention strategies that target the healthcare system and patients.
Diabetes Quality Improvement Interventions Among Underserved and Minority Populations
Glazier et al. conducted a systematic review of 17 studies examining the effectiveness of patient, provider, and health system interventions among patients with type 1 or type 2 diabetes in socially disadvantaged populations, defined as those of low socioeconomic status, or belonging to an ethnic/racial minority group [35]. Eight of 13 studies showed improvements in HbA1c but less impact on body weight, lipids, and blood pressure (see Table 14.3).
Table 14.3
Improvement in diabetes-related metabolic and process measures in response to patient, provider, and health system quality improvement interventions in socially disadvantaged populations [35]
Metabolic or process measure | # of studies with improvement/total # of studies examining measure |
|---|---|
HbA1c | 8/13 |
Weight/body mass index | 2/9 |
Lipids | 2/7 |
Blood pressure | 2/4 |
Adherence to annual eye exam | 3/3 |
Achievement of ADA care indicators | 2/3 |
Adherence to exercise recommendations | 1/3 |
Improved diabetes knowledge | 2/2 |
Improved physician trust | 1/1 |
Features of effective interventions included cultural and health literacy tailoring, led by community educators or lay people; 1:1 (versus group) interventions with individualized assessment/reassessment; incorporation of treatment algorithms, focusing on behavior-related tasks and providing feedback; and high intensity interventions over a long duration [35]. With one exception, interventions targeting racial/ethnic minorities specifically did not involve endocrinology subspecialty input on the care team. One study of NHBs and Latinos involved nurse implementation of a detailed protocol and algorithm with endocrinologist supervision which resulted in a significant 3.5 % reduction in HbA1c and improvement in 8/10 ADA process measures [36]. Components of successful interventions from this review are summarized in Table 14.4.
Table 14.4
Summary of components of successful diabetes quality improvement interventions in socially disadvantaged populations
Intervention level | Successful components |
|---|---|
Patient | Interpersonal |
Utilize social networks | |
Culturally tailored | |
Provider | In-person feedback (compared to computerized decision support) |
Health system | Utilize on-site nurse case managers |
Utilize community health workers | |
Provide medical assistance programs for prescriptions |
Interventions specifically targeting NHBs and Latino Americans with diabetes were recently summarized in two systematic reviews and meta-analyses [37, 38]. Table 14.5 below summarizes the types of interventions employed in these studies and the effect on process measures and clinical outcomes.
Table 14.5
Quality improvement intervention components in studies of predominantly minority populations
Intervention target(s) | Intervention component(s) | Summary of outcomes |
|---|---|---|
General patient level interventions | ||
Basch (1999) [39] (100 % NHB) | Education and counseling | ↑ Receipt of dilated eye exam |
Clancy (2003) [40] (78 % NHB) | Education and stress management (MD and RN group visits) | Improvement in testing of HbA1c, LDL, microalbumin; use of ACE inhibitors, ASA, statins, vaccinations, and eye and foot exams |
Increased physician trust | ||
Gerber (2005) [41] (66 % Latino, 29 % NHB) | Computer training on skills and self-management support in waiting room kiosk | No significant change in HbA1c, BP, weight, knowledge, self-efficacy |
Erdman (2002) [42] (91 % NHB) | Self-management, lifestyle counseling | ↓ HbA1c, total cholesterol, LDL (due to statins) |
↑ HDL | ||
Ziemer (2003) [43] (90 % NHB) | Diet counseling | ↓ HbA1c |
Anderson (2003) [44] (100 % NHB) | Patient reminders to schedule eye exam | ↑ Returning for annual diabetes retinal exam |
D’Eramo-Melkus (2004) [45] (100 % NHB) | Culturally tailored CBT and monthly nurse practitioner visits | ↓ HbA1c, weight, BMI, diabetes-related emotional distress |
Tang (2005) [46] (100 % NHB) | Patient-centered DSME | ↓ BMI, dyslipidemia |
Improved self-management behaviors, quality of life, difficulty with diet, difficulty with exercise | ||
Amoak (2008) [47] (100 % NHB) | Phone calls focusing on aspects of experience with diabetes administered by geriatric NP | Improved psychological adjustment and exercise |
Skelly (2009) [48] (100 % NHB) | Teaching and counseling modules delivered by RN in patient’s home | No change |
Tang (2010) [49] (100 % NHB) | DSME groups | ↓ HbA1c, BMI, dyslipidemia |
Walker (2010) [50] (100 % NHB) | Educational sessions with patient navigator follow-up | Improved diabetes knowledge |
Carter (2011) [51] (100 % NHB) | Online DSM | ↓ Weight, BMI, HbA1c |
Improved diabetes knowledge, diabetes management practices, physical health status, mental health status | ||
Ellish (2011) [52] (100 % NHB) | Eye exam newsletter | No change |
Tang (2012) [53] (100 % NHB) | Empowerment-based DSM support | ↓ Blood pressure, serum cholesterol |
Improved self-management behaviors, quality of life | ||
Culturally tailored patient level interventions | ||
Agurs-Collins (1997) [54] (100 % NHB) | Dieticians and medical staff support for weight loss | ↓ HbA1c, weight, blood pressure |
Anderson-Loftin (2005) [55] (100 % NHB) | Nutrition counseling by RN and DM educator | ↓ BMI, improved food habits |
DSM education and support group by bilingual Mexican American nurses, dietitians, and CHWs | ↓ HbA1c | |
Brown (2005) [58] (100 % Mexican American) | Extended DSM education support/education (vs. compressed education/support) | No difference in HbA1c |
Corkery (1997) [59] (75 % Puerto Rican; 25 % other Latinos) | Diabetes education via bicultural CHWs and diabetes RN educator (vs. diabetes RN educator) | Better program completion rate |
No difference in knowledge, behaviors, or HbA1c | ||
Keyserling (2002) [60] (100 % NHB) | CHW/peer counselors + nutritionist | ↑ Physical activity and diabetes knowledge |
Diet and physical activity intervention | ↓ Weight, BMI, fasting blood glucose (2001) | |
McNabb (1993) (100 % NHB) [63] | Diet and exercise education | ↓ Weight |
Rosal (2005) [64] (80 % Puerto Rican low-income, Spanish speaking) | Diet, physical activity, DSME education led by DM nurse, nutritionist, and CHW | ↓ HbA1c |
Non-sustained ↓ depressive symptoms | ||
No change in physical activity | ||
↑ Self-monitoring | ||
Two Feathers (2005) [65] (64 % NHB; 36 % Latino) | CHW lifestyle interventions | Improved dietary knowledge and behaviors and physical activity knowledge |
↓ HbA1c | ||
Vazquez (1998) [66] (100 % Caribbean American Latinos) | Nutrition program with nutritionists and psychologists | ↓ Caloric, total fat, total saturated fat, total energy intake |
↑ Carbohydrate intake | ||
Anderson (2005) [67] (100 % NHB) | Self-management experiments, problem-solving, discussing emotional experience living with diabetes delivered by RN and dietician | ↓ HbA1c, serum cholesterol, LDL, triglycerides, weight |
↑ HDL, SMBG | ||
Improved perceived understanding of diabetes, diabetes empowerment, attitudes toward seriousness of diabetes, positive attitudes | ||
Murrok (2009) [68] (100 % NHB) | Dance classes choreographed to gospel music | ↓ Body fat and blood pressure |
Bogner (2010) [69] (100 % NHB) | Individualized program to improve adherence to oral hypoglycemic and |antidepressants (recognizing social and cultural context) | ↓ HbA1c |
Improved adherence to oral hypoglycemics | ||
D’Eramo (2010) [70] (100 % NHB) | Culturally relevant group DSME training, coping skills training, and diabetes care intervention | ↓ HbA1c, blood pressure, dyslipidemia |
Improved quality of life, vitality, role physical, bodily pain, perceived provider support for diet, exercise, diabetes-related emotional distress | ||
Hill-Briggs (2011) [71] (100 % NHB) | Problem-based DSM training | ↓ HbA1c |
Improved knowledge, problem-solving, self-management behavior | ||
Provider level interventions | ||
Benjamin (1999) [72] (40 % NHB, 36 % Latino) | Provide based learning methods to increase MD use of practice guidelines | Improvement in annual eye and microalbumin exams |
Trends of improvements in annual lipids, influenza vaccines, and diet/diabetes education | ||
↓ HbA1c | ||
Din-Dzietham (2004) [73] (100 % NHB) | CME and guideline distribution (continuous QI program targeting providers) | Positive change in prevalence in selected patterns of care |
Fox and Mahoney (1998) [74] (100 % NHB) | Chart audit and feedback | Improvement in HbA1c, self-management behavior, eye exams, and vaccinations |
Computerized decision-support reminders, bimonthly in-person individualized feedback | ↓ HbA1c | |
Thaler (1999) [77] (100 % NHB) | Rapid turnaround HbA1c in one group; both groups seen by nurse practitioner and endocrinologist | ↓ Fasting or random plasma glucose |
↑ Frequency of therapy intensification by treatment modality | ||
Ziemer (2006) [78] (100 % NHB) | Computerized reminders to intensify medications, reminders | Improvement in medication titration by providers; therapy intensification associated with ↓ HbA1c |
Health system | ||
Thaler (1999) [77] (100 % NHB) | Rapid turnaround HbA1c in one group; both groups seen by nurse practitioner and endocrinologist | ↓ Fasting or random plasma glucose |
↑ Frequency of therapy intensification by treatment modality | ||
RN case management, DM registry, visit reminders | ↓ HbA1c | |
Davidson (2003) [36] (86 % Latino) | RN-directed care with treatment algorithms | Improved HbA1c, lipid, annual eye exams, renal tests, foot exams, education, diet counseling |
↓ HbA1c | ||
Fanning (2004) [81] (100 % NHB) | RN case management (university and community settings) | ↓ HbA1c, LDL, blood pressure |
Gary (2004) [82] (100 % NHB) | RN case management, CHW | ↓ Blood pressure, lipid profile |
Hopper (1984) [83] (75 % NHB) | Home health aide | ↓ Fasting blood sugar (among those offered aides) |
↑ Eye clinic visits among those who accepted aides | ||
Hosler (2002) [84] (45 % NHB, 25 % Latino, 7 % Native American) | Evidence-based guidelines, multidisciplinary DM team, minority outreach, community partnerships (CHWs, patient incentives) | Improved assessment of HbA1c, lipids, eye exam, and SMBG, exercise, and diet assessment |
Jaber (1996) [85] (100 % NHB) | Pharmacist-led education | ↓ HbA1c and fasting glucose |
Miller (2003) [86] (100 % NHB) | Rapid turnaround HbA1c | ↓ HbA1c |
↑ Frequency of pharmacological diabetes therapy | ||
Philis-Tsimikas (2004) [87] (72 % Latino) | RN case management with treatment algorithms + CHW | 100 % compliance with ADA standards for performing HbA1c, lipids, foot exams, and urine microalbumin |
↓ HbA1c, cholesterol, blood pressure | ||
Improved DM knowledge and self-efficacy | ||
Multi-target interventions (patients and healthcare system) | ||
Cook (1999) [88] (88 % NHB) | Patient education, nurse case management, multidisciplinary diabetes team (including endocrinologist), treatment algorithms | ↓ HbA1c |
↑ BMI | ||
Anderson-Loftin (2002) [55] (100 % NHB) | Culturally competent, dietary self-management intervention with dietician and nurse case manager | ↓ HbA1c, fasting blood glucose, frequency of acute care visits |
Improved dietary habits | ||
Bray and Thompson (2005) [79] (100 % NHB) | RN case management (APN) with supervising physician (unclear if endocrinologist), DM registry, visit reminders, patient education and self-management support, provider decision-support tools | ↓ HbA1c |
Mahotiere (2006) [89] (100 % NHB) | Provider QI interventions focused on system changes surrounding the physician visit combined with patient interventions | ↑ proportion of beneficiaries with diabetes receiving biennial lipid profile |
Gary (2009) [90] (100 % NHB) | Nurse case management and CHW using evidence-based clinical algorithms with feedback to PCPs | ↓ ED visits and blood pressure |
↑ HDL | ||
Thaler (1999) [77] (100 % NHB) | Rapid turnaround HbA1c in one group; both groups seen by nurse practitioner and endocrinologist | ↓ Fasting or random plasma glucose |
↑ Frequency of therapy intensification by treatment modality | ||
Rith-Najarian (1998) [91] (100 % NHB) | Podiatric screening and patient education, provider guidelines, multidisciplinary team, DM registry and tracking system, flow sheets | ↓ Amputations |
Patient Interventions Within Healthcare Organization
In Peek’s review of 17 studies of patient interventions within the healthcare organization that sought to improve dietary habits, physical activity, and self-management activities, those that were culturally tailored were more effective in lowering HbA1c than general QI interventions (−0.69 % versus –0.1 %) [37]. Also, peer support and 1:1 interventions were more effective than online and computer-based approaches. In a systematic review and meta-analysis looking exclusively at randomized controlled trials of patient interventions targeting NHBs, most of which were culturally adapted and included peer providers, two of 22 increased patient attendance at screening visits, and 20 of 22 promoted diabetes self-management [38]. In a meta-analysis of eight studies, interventions resulted in a significant 0.83 % reduction in HbA1c [38].
Provider Interventions
In Peek’s review, provider interventions including education, continuing medical education, computerized decision support, in-person feedback, and problem-based learning improved process measures [37]. The majority of these studies were conducted in NHBs with diabetes. Interventions involving computerized decision-support reminders and chart audit and individual’s feedback resulted in improved HbA1c and treatment modification [74–76, 92].
Healthcare Organization Interventions
Healthcare organization interventions in minority populations have included systems for rapid turnaround HbA1c, circumscribed appointments, support staff (e.g., nurse case management, community health worker, pharmacist), and increased follow-up through home visits or telephone/mail contact [37, 38]. In Peek’s review, 14 studies with interventions targeting the healthcare organization resulted in a mean HbA1c reduction of 0.34 %. Ricci-Cabello et al. included five healthcare system intervention trials in NHBs in their systematic review and meta-analysis and found the two most highly effective interventions in improving HbA1c, and frequency of therapy intensification included rapid turnaround HbA1c [77, 86].
Multi-target Interventions
Multi-target interventions target all aspects and components of healthcare delivery, including patients, providers, and the healthcare system. Five of these studies have targeted NHBs with diabetes and used various approaches. Three studies showed an improvement in HbA1c [55, 79, 88]. All of these interventions included patient education and self-management support and nurse case management, two included treatment algorithms [79, 88], and two involved collaboration with a physician in treatment decisions (one an endocrinologist [88] and one an unspecified physician [79]). While two additional multi-target interventions showed improvement in process measures and non-glycemic clinical outcomes [89, 90], they did not improve glycemic control. One study involved patient interventions and provider-focused QI interventions focusing on system changes surrounding the physician visit [89] and the other involved nurse case management and community health workers using evidence-based clinical algorithms with feedback to primary care physicians [90]. Finally, one study focusing exclusively on Native Americans in the Indian Health Service included provider guidelines, a multidisciplinary team, diabetes registry/tracking system, and flow sheets [91]. Compared to podiatric screening and patient education, the multi-target intervention resulted in a significant reduction in amputation rate [91].
Non-pharmacological Management of Diabetes in Minority and Underserved Populations
Lifestyle intervention is the mainstay of non-pharmacological management of diabetes. The American Diabetes Association recommends initial management of diabetes with 6 months of diabetes self-management education (DSME) to support lifestyle modifications to achieve modest weight loss. The physical activity and dietary recommendations are >150 min of moderate-intensity physical activity, and medical nutrition therapy emphasizing a variety of nutrient-dense foods in appropriate portion sizes focused on reduced energy intake for individuals with a BMI ≥25 kg/m2 with proper macronutrient distribution [93]. In addition to physical activity and dietary management, novel factors for diabetes management have emerged including dietary patterns, dietary composition, intensity of physical activity, and weight loss with both nonsurgical and surgical approaches.
Dietary Patterns
Mediterranean Diet
The largest analysis of the Mediterranean diet was the Prevention with Mediterranean Diet (PREDIMED) study. The trial enrolled 7,447 persons in Spain at high cardiovascular risk with either diabetes or three cardiovascular risk factors. Participants were randomized to one of three diets: a Mediterranean diet supplemented with extra-virgin olive oil, a Mediterranean diet supplemented with mixed nuts, or a control diet (advise to reduce dietary fat) and were followed for 4.8 years for the development of the primary end point of major cardiovascular events. Mediterranean diet with extra-virgin olive oil and Mediterranean diet with nuts were associated with 30 and 28 % reductions in major cardiovascular events [94]. Among participants with diabetes in the Mediterranean diet groups compared to control, there was a 29 % reduction in major cardiovascular events. The Mediterranean diet also reduces HbA1c. In 215 overweight Italian individuals with newly diagnosed diabetes, Mediterranean diet vs. low-fat diet was associated with a 0.6 % and 0.4 % lower HbA1c at 1 and 4 years, respectively, with a 37 % reduction in antidiabetic medication use [95]. In 259 Israeli participants, a low-carbohydrate Mediterranean diet versus a traditional Mediterranean diet or American Diabetes Association diet was associated with greater reductions in HbA1c and cardiovascular risk factors [96]. Notably, the majority of the improved glucometabolic findings with the Mediterranean diet remained significant with adjustment for weight loss. Although, the Mediterranean diet has not been assessed in a clinical trial among US ethnic minorities, observational findings in the Multiethnic Study of Atherosclerosis (MESA) (42 % white (NHW), 12 % Chinese American (CA), 26 % NHB, 21 % Hispanic American) reveal that participants with greater adherence to a Mediterranean dietary pattern had lower insulin and glucose independent of racial/ethnic group [97]. These results suggest a likely beneficial effect of the Mediterranean diet on improved glucose metabolism among US racial/ethnic minorities with diabetes, although intervention trials in these populations are needed.
Vegetarian Diet
The Adventist Health Study 2 assessed the role of vegan and vegetarian diets in the USA. Among 73,308 NHB and NHW participants with no history of cancer or cardiovascular disease, there was a 12 % reduction in all-cause mortality over 5.8 years [98], 30–50 % lower diabetes prevalence [99], and 40–60 % lower diabetes incidence in the vegetarian versus non-vegetarian groups [100]. These findings lend credence to improved glucose metabolism with vegetarian dietary patterns. In individuals with diabetes, a small (n = 99) multiethnic (NHW 44 %, NHB 44 %, Hispanic American 4 %, and Asian American 8 %) clinical trial of a low-fat vegan diet or American Diabetes Association diet for 72 weeks revealed greater reductions in HbA1c and cardiovascular risk factors in the low-fat vegan group [101].
DASH Diet
The Dietary Approaches to Stop Hypertension (DASH) diet encourages the intake of fruits, vegetables, whole grains, and low-fat dairy products in combination with sodium restriction [102]. The DASH diet was initially studied in a multiethnic trial with 65 % ethnic minorities (35 % NHW, 60 % NHB, 5 % other minority groups) and significantly lowered blood pressure among all participants with twofold greater reductions among racial/ethnic minorities [102]. Further studies revealed that components of the DASH diet may also improve insulin sensitivity [103]. The DASH diet is recommended for all Americans including those with diabetes [93].
Dietary Composition
Glycemic Index/Glycemic Load
Carbohydrates are the chief dietary component influencing insulin secretion and postprandial glycemia [104]. The glycemic index measures the effect of carbohydrates on postprandial blood glucose, and the glycemic load takes into account the glycemic index and the amount of carbohydrate consumed. Several studies with multiethnic participants using low-glycemic index eating patterns have demonstrated HbA1c decreases (0.2–0.5 %), but these are not unanimous [105]. One difficulty is that fiber intake was not consistently controlled, thereby causing difficulty in interpretation. Results of low-glycemic index/glycemic load diets on cardiovascular disease risk factors including low-density lipoprotein and total cholesterol are mixed [105].
Low-Carbohydrate/Low-Fat Dietary Approaches
Low-carbohydrate versus low-fat diets have been extensively examined in diabetes management. A 2012 meta-analysis of 23 clinical trials of low-carbohydrate versus low-fat diets including studies of participants with and without diabetes revealed that both diets reduced glucose in a similar manner [106]. We will focus on data from two recent low-carbohydrate versus low-fat diet trials in overweight or obese patients with diabetes. First, a 12-month multiethnic trial (59 % NHB, 3 % Hispanic American, 38 % NHW) of obese (BMI ≥35 kg/m2) adults was randomized to low-carbohydrate (LC) (<30 g/day) or a calorie-restricted low-fat diet (LF) (500 calorie reduction with <30 % calories from fat). Among 54 participants with diabetes, HbA1c decreased by 0.7 % in the LC group versus 0.1 % in the LF group (p = 0.02) with no significant weight-loss differences [107]. Second, a 12-month multiethnic trial (64 % NHB, 16 % Hispanic American, 15 % NHW, and 3 % Asian) of 105 overweight participants with diabetes randomized to a LC versus LF diet revealed no significant difference in weight (–3.4 %) or HbA1c change (−0.02 % LC versus 0.24 % LF, nonsignificant) [108]. A major strength of these studies is the inclusion of ethnic minorities, but a weakness is the inability to stratify by race due to power limitations, so it is unclear whether there are racial/ethnic differences. Based on these studies, the potentially greater HbA1c reduction with LC vs. LF diets appears to be independent of weight loss.
Physical Activity and Exercise
Exercise interventions improve glycemic control in diabetes in majority of NHW studies [109]. A meta-analysis examining the effects of exercise interventions (mean 3.4 sessions/week with 49 min/session) on glycemic control in individuals with diabetes revealed mean HbA1c reduction of 0.66 % (p < 0.001) in people with diabetes, even with no significant change in BMI [109]. Higher levels of exercise intensity are associated with greater improvements in HbA1c and fitness [110]. Clinical trials have provided strong evidence for the HbA1c lowering value of resistance training in NHW older adults with type 2 diabetes [111]. There are limited data on glycemia in response to exercise in NHB, Hispanic Americans, and Native Americans with diabetes. Among individuals without diabetes in the HERITAGE Family Study, a 20-week trial of thrice weekly exercise in NHB and NHW, NHB had a greater increase in fasting glucose with exercise compared to NHW, although there were reductions in fasting insulin in both groups [112]. A multiethnic (52.7 NHW, 43.5 % NHB, 3.8 % Hispanic American) randomized clinical trial examining the effect of aerobic exercise, resistance training or combined aerobic exercise, and resistance training on HbA1c levels in 221 participants with diabetes found that aerobic exercise alone or resistance training alone had no effect on HbA1c, only the combined group revealed significant reductions in HbA1c (−0.34 %, p = 0.03) and improvements in cardiorespiratory fitness over 9 months [113]. They lacked the power to examine racial/ethnic differences. Potential components of improved glucose metabolism with physical activity are skeletal muscle mitochondrial mass, mitochondrial function, and aerobic capacity [114, 115]. The racial/ethnic differences of aerobic capacity with physical activity were tested in a 6-month trial of aerobic exercise training in NHB and NHW postmenopausal women. The NHW women had a greater increase in cardiorespiratory fitness with sustained resting metabolic rate, whereas in NHB women, resting metabolic rate declined over the course of the trial, suggesting a perturbation in mitochondrial function [116, 117]. A study of exercise capacity and all-cause mortality in NHB and NHW men with diabetes found that exercise capacity is a stronger and more graded predictor of mortality for NHW than for NHB men [118]. The high-fit compared to low-fit NHW and NHB men had a 67 % and 46 % reduction in mortality risk, respectively [118]. These studies reveal that there is a benefit to physical activity in US racial/ethnic minorities, but the type (aerobic vs. resistance) and intensity of the physical activity need to be further characterized. A recent meta-analysis of step counters to improve physical activity and HbA1c in individuals with diabetes underscores this point. Data from 11 randomized clinical trials using step counters (pedometers) revealed that step counter use is associated with a significant increase in physical activity (1,822 steps/day), but no change in HbA1c [119].
Weight Reduction: Bariatric Surgery/Lifestyle Interventions
Elevated body mass index and adiposity are the most important predictors of diabetes [120–122]. Obesity is also an independent predictor of clinical cardiovascular disease [123–125]. Mortality for diabetes, myocardial infarction, and stroke was similar for each of these conditions, but when any two are combined, the risk is multiplicative [126], reinforcing the importance of improving weight in individuals with elevated weight. The underlying relationship between obesity, cardiovascular disease, and diabetes has been reviewed and is potentially mediated through the adipokines released from visceral fat [127–130]. In this section we will review the data on nonsurgical and surgical interventions to promote weight loss in diabetes and improve glycemia with the goal of long-term reduction in morbidity and mortality.
Lifestyle, Diet, and Behavioral Interventions
The largest analysis of a lifestyle intervention in individuals with diabetes was Look AHEAD [131], a multiethnic (63 % NHW, 16 % NHB, 13 % Hispanic American, 5 % Native American, 1 % Asian or Pacific Islander, and 2 % other minorities) randomized controlled lifestyle intervention trial with 5,145 overweight or obese participants. Participants were randomized to either intensive lifestyle intervention that promoted weight loss through decreased caloric intake and increased physical activity (intervention group) or received diabetes support and education (control group) and followed a median of 9.6 years for the development of the primary outcome of a composite of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina. They found no association between the lifestyle intervention and primary outcome (HR: 0.95, p = 0.51). Notably, the hazard ratios for Hispanic Americans, NHBs, NHWs, and Native Americans were 0.66 (95 % CI: 0.41–1.05), 1.34 (95 % CI: 0.91–1.96), 0.94 (95 % CI: 0.80–1.11), and 0.74 (95 % CI: 0.31–1.76), suggesting possible variance by race/ethnicity, especially for Hispanic Americans (HA) compared to NHBs. The authors and others note that differences in rates of cardioprotective medication use and power issues due to lower than expected cardiovascular disease event rates may account for the null finding. There was also a suggestion of heterogeneity of response to intervention based on the history of cardiovascular disease at baseline (p = 0.06). There were a number of positive outcomes of the study including reduced hepatic steatosis, body weight, healthcare costs, and microvascular outcomes including diabetic nephropathy and retinopathy, as well as increased physical fitness, physical function, glucose control, and quality of life [132–134]. Given the benefit in these secondary outcomes from a diabetes management perspective, there are many important reasons to encourage overweight and obese individuals with diabetes to enroll in a lifestyle intervention program with the goal of weight loss [134].
In a multiethnic (59 % NHB, 32 % NHW, 2 % Hispanic American) randomized comparison of a commercially available portion-controlled weight-loss intervention with a diabetes self-management education program, the portion-controlled group lost greater weight (7.3 kg vs. 2.2 kg) with a larger reduction in HbA1c (0.7 % vs. 0.4 %) [135]. Finally, a recent systematic review and network meta-analysis of 33 studies (4,774 participants) evaluating behavioral programs in diabetes management revealed that in comparison with usual care among ethnic minority participants, behavioral programs lowered HbA1c by 0.42 % compared to usual care [136].
Surgical Interventions
Bariatric Surgery for Individuals with BMI ≥35 kg/m2
Bariatric surgery procedures are associated with diabetes remission due to hormonal changes and weight loss [137]. Hormonal changes include reduction in ghrelin and increases in glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP). Lower levels of ghrelin postsurgically decrease appetite stimulation through a decrease in stimulation of orexigenic neurons neuropeptide Y/agouti-related peptide. Postsurgically levels of glucagon-like peptide-1 and glucose-dependent insulinotropic peptide increase exerting effects on the enteroinsular axis with potent insulinotropic activity. The long-term weight loss occurs due to decreased food (energy) consumption caused by the reduction in appetite and anatomical constraints. The surgical procedures currently performed include laparoscopic adjustable gastric banding (LAGB), laparoscopic vertical sleeve gastrectomy (LVSG), Roux-en-Y gastric bypass (RYGB), biliopancreatic diversion, and biliopancreatic diversion with duodenal switch (Fig. 14.1). In the USA, RYGB was the most commonly performed procedure but with wide adoption of LVSG; LVSG has surpassed RYGB and is performed in one half of bariatric surgical procedures [139]. The type of procedure is important for individuals with diabetes as diabetes remission varies greatly across procedures. Meta-analyses and reviews report remission rates of 48 % (29–67 %) for LAGB, 84 % (77–90 %) for RYGB, 72 % (55–88 %) for LVSG, and 99 % (97–100 %) for biliopancreatic diversion with duodenal switch [140, 141]. Recent analysis of the two most popular procedures have shown diabetes remission rates ranging from 27 to 75 % for LVSG versus 42–93 % for RYGB [142]. In the STAMPEDE trial, diabetes remission rates at 1 and 3 years of follow-up were 12 and 5 % with intensive medical therapy alone, 42 and 38 % in RYGB group, and 37 and 24 % in the LVSG group (Table 14.6). The diminishing rates of diabetes remission over time noted in STAMPEDE are also seen in other studies of LVSG and RYGB at a rate of 7–15 % per year [137]. Given the current popularity of LVSG, longer-term comparative effectiveness data on LVSG are needed. However, bariatric surgery experts believe the effect of LVSG on weight loss and comorbidity improvements seems to be somewhere between those of RYGB and LAGB [151]. Five-year outcomes from RYGB among mostly NHW participants found that shorter diabetes duration, lower HbA1c, younger age, higher serum insulin levels, and nonuse of insulin were associated with higher remission rates [152].
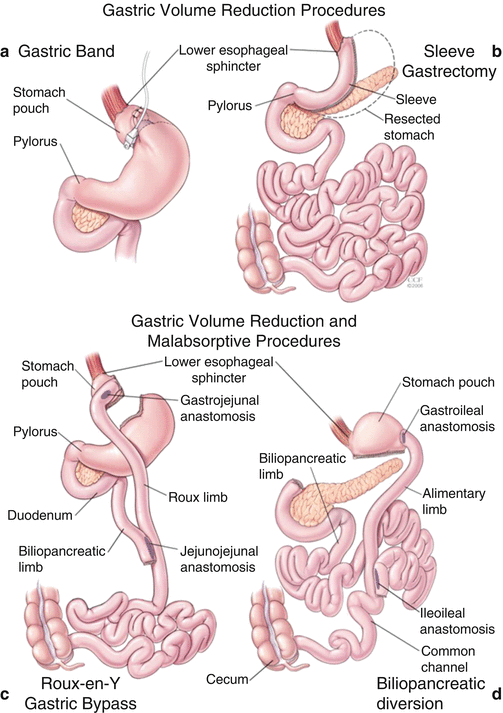

Fig. 14.1
Anatomic modifications as created in the four most common bariatric surgery procedures. (a) Gastric banding involves placement of an adjustable gastric band around the proximal part of the stomach. The band is fixed in position by inserting plication sutures anteriorly. The band can be adjusted/tightened over time by injecting fluid into the subcutaneous port connected to the band. (b) Sleeve gastrectomy involves reducing gastric volume by 75–80 % by resecting the stomach alongside a 30 F endoscope beginning 3 cm from the pylorus and ending at the angle of His. (c) The Roux-en-Y gastric bypass involves creation of a 15- to 20-mL gastric pouch, a 150-cm Roux limb, and a 50-cm biliopancreatic limb. (d) The biliopancreatic diversion procedure includes a distal gastrectomy with long Roux-en-Y reconstruction, where the enteroenterostomy is placed ≈50 cm proximal to the ileocecal valve. Both the volume of the gastric remnant and the length of the alimentary limb can be modified to suit the patient’s weight loss goal (Vest et al. [138])
Table 14.6
Glycemia, diabetes remission, cardiovascular risk factors, and cardiovascular events in diabetes bariatric surgery studies
Publication year | Study/site/size | Population characteristics/ethnicity | Study design/interventions
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|