Fig. 3.1
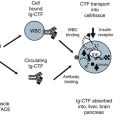
Adiponectin Receptor System. In normal patients, the adiponectin receptor system allows adiponectin to reduce glucose production in the liver (AdipoR2) and causes glycolysis and fatty acid oxidation in muscle (AdipoR1) through signaling cell metabolism via 5′-AMP activated protein kinase (AMPK). Adiponectin also activates cell growth (MAPK p38) and anti-atherogenic responses (PPAR α). AdipoR2 reacts to full length adiponectin in low, medium and high molecular weight forms but not globular adiponectin (gAdpn). AdipoR1 is more responsive to gAdpn. In metabolic syndrome patients, adipocytes become hypertrophic due to lipid accumulation. The result is less adiponectin is produced and a reduced the AMPK response in muscle and liver. In the liver heptocytes, glucose production is not shut down and in the muscle myocytes, less glycolysis and fatty acid oxidation occurs
The clinical sensitivity of Adpn to diagnose and monitor diabetes is poor with many false negatives and positives. A greater understanding of the Adpn pathway is needed to improve clinical accuracy. It is unknown how Adpn impacts insulin sensitivity. Adpn alone has no effect on protein kinase B (Akt) phosphorylation (Tsao et al. 2002). There is also no explanation for Apdn inflammatory behaviors. Adpn inhibits tumor necrosis factors -α (TNF-α) induced adhesion molecule expression, inhibits nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation in endothelial cells and reduces macrophage production OD TNF-α (Ouchi and Walsh 2007; Yamauchi et al. 2003). Adpn also activates mitogen-activated protein kinase (MAPK p38) and induces endothelial apoptosis (Bråkenhielm et al. 2004).
3.1.2 Role of Adiponectin Receptor
The adiponectin receptor is a membrane bound G protein-coupled receptor with seven transmembrane domains; the N-terminal in the cytosol and the C-terminal in an unexpected extra-cellular position (See Fig. 3.2). Two adiponectin receptors have been identified. AdipoR1 is ubiquitously expressed and abundant in skeletal muscle. AdipoR2 is expressed mostly in the liver (Kadowaki et al. 2003, Yamauchi Kadowaki et al. 2003; Bråkenhielm et al. 2004). T-cadherin is a novel receptor found for high-molecular-weight (HMW) adiponectin multimers (Hug et al. 2004). The internal N-terminus of AdipoR1 was found to interact with the adaptor protein containing a pleckstrin homology domain, phosphotyrosine binding domain, and leucine zipper motif to initiate the downstream cascade AMPK (Ouchi and Walsh 2007). The external C-termini is of unknown function. It is unclear if Adpn interacts with the C-terminus or the loops between the transmembrane domains.
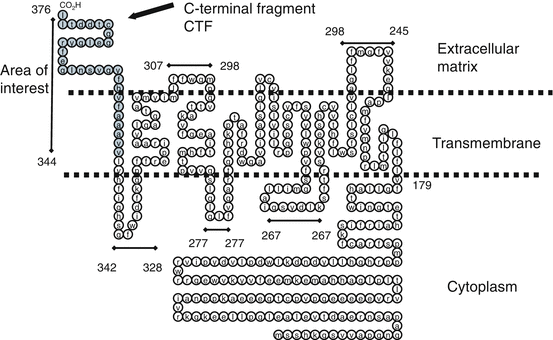

Fig. 3.2
AdipoR1 inverted G protein-coupled receptor. The adiponectin receptor is an inverted membrane bound G protein-coupled receptor with seven transmembrane domains and the N-terminal in the cytosol and the C-terminal in an unexpected extra-cellular position. The C-Terminal for G protein-coupled receptors is typically on cytoplasm side
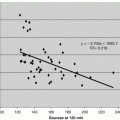
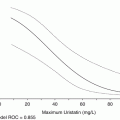
Our goal was to investigate the function of the C -terminal fragment (CTF) as a potential new avenue in this mechanism. We previously reported that the C-terminal fragments of adiponectin receptor (named as AdipoR CTF) existed in human plasma, and the free CTF was low or missing in most diabetic patients tested using immuno-affinity mass spectroscopy (Pugia et al. 2009). CTF was significantly higher (P > 0.001) in non-diabetics (n = 10, ave 3.1 ng/ml, sd 0.6) than diabetics (n = 10, ave 0.4 ng/ml, sd 0.1). This chapter demonstrates methods used to translate a proteomic discovery into a clinical assay. We report methods for measurement of Adiponectin receptor (AdipoR) in blood which offer tools to for researches to further explore this pathway (Pugia 2012; Pugia and Ma 2013).
3.2 Methods
3.2.1 AdipoR1 and R2 CTF Peptide Preparation
Peptides were made to match the clinical form, to cover the cleavage site, to serve as soluble standards, to produce antibodies and to study the inhibition of proteases. (See Table 3.1 and supplemental table on CTF peptide sequences). AdipoR1 and R2 peptides were either synthesized by Siemens Healthcare Diagnostics (Walpole MA) or under contract to Celtek Biosciences LLC (Nashville TN). The 17 mers were obtained from Phoenix Pharamaceuticals Inc (Belmont, CA). All peptides were characterized by an HPLC C-18 column and matrix-assisted laser desorption/ionization (MALDI) mass spectroscopy and typically >90 % pure.
Table 3.1
Adiponectin receptor C- terminal fragment peptides
Peptide name | Number of amino acids (mer) | Description of peptide |
|---|---|---|
AdipoR1 CTF343-375 | 33 | R1 Longer form |
AdipoR1 CTF344-375 | 32 | R1 Clinical form |
AdipoR2 CTF344-375 | 32 | R2 Clinical form |
AdipoR1 CTF343-375 | 33 | Used for polyclonal antibodies |
AdipoR1 CTF341-370 | 30 | Covers the cleavage site |
AdipoR1 CTF341-355 | 15 | Covers the cleavage site |
AdipoR1 CTF351-375 | 25 | R1 Soluble portion |
AdipoR2 CTF351-375 | 25 | R2 Soluble portion |
AdipoR2 CTF351-375 | 13 | R2 used for standard |
AdipoR1 CTF358-375 | 17 | Non inhibitory domain |
AdipoR2 CTF358-375 | 17 | Non inhibitory domain |
AdipoR1 CTF351-362 | 12 | Inhibitory domain (active site) |
AdipoR1 CTF344-353 | 10 | Non inhibitory domain |
AdipoR1 CTF367-375 | 9 | Tail (end of C terminal) |
AdipoR2 CTF367-375 | 9 | Tail (end of C terminal) |
The peptides matching the masses in human clinical blood were previous identified by immune-affinity mass spectroscopy techniques as the 32 mers of AdipoR1 CTF343-375 (Pugia et al. 2009). These 32 mer peptides had poor solubility due to Val-Val-Ala-Ala (Gly)-Ala-Phe-Val sequence and therefore were of lower purity and difficult to work with. The 25 mer peptides AdipoR1 CTF351-375 had good solubility and were suitable materials for standards. This material was dissolved at 2 mg/mL in 60 % DMSO-water. All peptides containing the cysteine at position 269 readily dimerized unless dissolved in 60 % acetonitrile-water with 0.1 % TFA. Mixing peptides in the absence of trifluoroacetic acid (TFA) allowed the R1-R2, R1-R1 and R2-R2 25 mer dimers to be synthesized in high purity.
3.2.2 CTF Antibodies
Mouse monoclonal antibody (mAb) clones 444, 515, 510, and 461 were raised against R1 25-mer (soluble section), R1 9-mer (tail), R2 9-mer (tail) and R1 15-mer (cleavage site) peptides using standard methods (See Table 3.2). Biacore testing against the R1 and R2 peptides for 9-mer, 12-mer, 25-mer and 32-mers showed all mAb to be medium affinity except 515 which was low affinity. A high affinity rabbit monoclonal was made to R1 12-mer (active site) conjugated to keyhole limpet hemocyanin (KLH) carriers for immunization (Epitomics Inc. Burlingame, CA). Binding kinetics were measured using surface plasmon resonance (Biacore et al. 3000 instrument).
Table 3.2
Adiponectin receptor CTF antibodies & cross reactivity and pairing
Antibody | Binding site | Raised against | Clone identification |
|---|---|---|---|
Mouse mAb 444 R1CTF | Active site | AdipoR1 CTF351-375 | ATCC 444-1D12.1H7 PTA 12564 |
Mouse mAb 515 R1CTF | Tail | AdipoR1 CTF367-375 | ATCC 515-4D6.1B10 PTA 12563 |
Mouse mAb 510 R2CTF | Tail | AdipoR2 CTF367-375 | ATCC 510-6B6-1G1 PTA 12565 |
Mouse mAb 461 R1CTF | Cleavage site | AdipoR1 CTF341-354 | ATCC 461-4H11.2A4 PTA 12562 |
Rabbit mAb R1CTF | Active site | AdipoR1 CTF351-362 | SAT-56-1 IgG |
Rabbit pAb R1CTF | Soluble portion | AdipoR1 CTF351-375 | NA |
Rabbit pAb R1CTF | Active site | AdipoR1 CTF351-362 | NA |
Antibody | Reacted with | No cross reaction with | Pairing |
|---|---|---|---|
ATCC 444-1D12.1H7 | R1 12-, 25-, 32-mer peptides | R2 peptides or the R1 9-mer peptide | pAb against CTF 25- mer mAb 461 or 515 |
ATCC 515-4D6.1B10 | R1 9-, 25-, 32- mer peptides | R2 peptides or the R1 12-mer peptide | – |
ATCC 510-6B6-1G1 | R2 9-, 25-, 32- mer peptides | R1 peptides or the R2 12- mer peptide | – |
ATCC 461-4H11.2A4 | R1 32- mer peptide | R2 peptides or the R1 9-, 12- or 25- mer peptides | – |
Rabbit monoclonal | R1 12-, 25-, 32- mer peptides | R2 peptides or the R1 9- mer peptides | pAb against CTF 25- mer mAb 461 or 515 |
Rabbit polyclonal antibodies (pAb) were raised against CTF R1 33-mer and 25-mer using standard methods (See Table 3.2). Rabbits did not produce antibodies to 33 mers conjugated with bovine serum albumin (BSA) but did with conjugation to KLH. Both BSA and KLH conjugates to 25 mer produced antibodies. Cross reactivity and paring studies were conducted on all antibodies by ELISA (See Table 3.2). The sequence for rat was only one amino acid different and all clones cross-reacted.
3.2.3 Conjugated Antibodies and Standards
Conjugates were made for ELISA, Western blot (WB), immunohistochemistry (IHC) and immunocytochemistry (ICC) analysis. ALP and FITC conjugated antibodies were tested for uses and cross reactivity (See Table 3.3). Immunoglobulin CTF standard was made by conjugating AdipoR1 CTF375-351 and R2 CTF 375-363 to human IgG. AdipoR1 CTF375-351 was used as standard for free CTF ELISA. All materials were stored in 0.1 M Tris, 0.25 M NaCl, pH 7.3, 0.5 % BSA and 0.1 % NaN3.
Table 3.3
Antibodies conjugates and uses
Conjugates and standards | Binding site | Std | Pair with | Use | Assay for |
|---|---|---|---|---|---|
Anti- R1CTF pAb25 mer-ALP | Multiple | R1 25 mer | 444 | ELISA, WB | R1CTF |
Anti- R2CTF mAb510-FITC | Tail | – | – | IHC, ICC | R2 CTF Ig- R2CTF |
Anti- R1CTF mAb461-FITC | Cleavage site | – | – | IHC, ICC | R1CTF |
Anti- R1CTF mAb444-FITC | Active site | – | – | IHC, ICC | R1 CTF Ig- R1CTF |
Anti human IgG mAb to ALP | Fab | Ig-R1 CTF | 444 | ELISA | Ig- R1CTF |
Ig-R2 CTF | 510 | Ig- R2CTF | |||
Anti- R1CTF mAb444-ALP | Active site | – | – | WB | R1CTF Ig- R1CTF |
3.2.4 Western Blot Methods
Western methods for measuring CTF R1 or R2 and the related protein were done per standard denatured SDS PAGE gels or native gel techniques (See on-line supplement for full procedure at www.extras.springer.com/2015/978-3-319-21926-4). Low molecular weights peptides were analyzed with 20 % Tris -Tricine SDS PAGE gels. Transfer membranes were incubated with antibodies for CTF R1 or R2 conjugated to alkaline phosphate and developed with substrate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree