Seborrheic keratoses: stuck-on, waxy, crumbling, hyperpigmented papules and plaques.
Recognition and reassurance are all that is typically required when counseling patients. Although UV-induced signature mutations have been described in seborrheic keratosis and they have a predilection to arise in sun-exposed areas, they have no malignant potential. Treatment is sometimes sought when they become inflamed or pruritic. Irritation can occur spontaneously or due to friction from clothing as a result of their predisposition to occur along the waistline or in the inframammary folds in female patients. Inflamed seborrheic keratoses often have a pink or crusted appearance and may be confused for melanoma or non-melanoma skin cancer in sun-exposed areas, which occasionally leads to biopsy. Although no reliably useful topical treatments exist, cryotherapy, curettage, sharp removal, and a number of other destructive methods can be quite effective for lesions that are inflamed or cosmetically displeasing to patients.
Solar lentigines
The solar lentigo, also referred to as an actinic lentigo, is proliferation of melanocytes with increased melanin production limited to the basal layer of the epidermis. As the name implies, it is found with increased incidence in sun-exposed skin.[40] Although they have colloquially been referred to as “liver spots,” there is no relationship to hepatic disease. They are usually multiple on the shoulders, face, forearms, and dorsal hands, but can affect any surface with a history of natural or artificial UVR exposure. The pigmentation is typically relatively evenly distributed, and size can range from a few millimeters to 3 cm–4 cm in size. The borders often have a moth-eaten or slightly notched appearance.
Lentigines often darken with exposure to UV light but do not completely fade over time in its absence as is typical of ephilides (freckles). Lentigines are benign; however, the most challenging differential diagnosis is with lentigo maligna, as both can show subtle irregularities of pigment, slightly irregular borders, or sizes that are not typical for most benign melanocytic lesions.[41]
Actinic purpura
Actinic purpura, also referred to as senile or solar purpura, are found in approximately 12% of patients over the age of 50 years, and in up to one-third of elderly patients in long-term care facilities.[42] These asymptomatic, nonpalpable purpura are most common on surfaces routinely exposed to minor trauma, such as the forearms (Figure 35.2). As normal aging and UV light contribute to decreases in collagen and subcutaneous fat, the skin and microvasculature become more susceptible to the routine shearing and mechanical forces that occur with daily activities. More often than not, a specific trigger for a lesion cannot be recalled. The result is the leakage of red blood cells and formation of noninflammatory purpura.
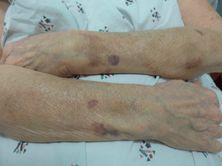
Actinic purpura: purpura classically located on the forearm with absence of yellow-green hues.
Typical resolution occurs over the course of three weeks without the evolution of yellow and green coloration seen in traumatic purpura. Persistent hyperpigmentation following repeated purpura may result due to hemosiderin deposition over time. Anticoagulants such as aspirin and warfarin can predispose patients to larger and more frequent purpura. Interestingly, decreases in dermal collagen resulting in actinic purpura may correlate to decreased bone collagen and osteoporosis.[43] Palpable purpura, especially on sites beyond the forearms, should raise suspicion of vasculitis. The presence of purpura on the trunk and proximal extremities that evolve with yellow and green coloration should trigger concern for elder abuse.
Other common benign skin lesions
Sebaceous hyperplasia
Sebaceous hyperplasia is commonly noted on the face of elderly patients and occasionally confused with basal cell carcinoma. These symmetric yellow papules typically have a central punctum from which a small droplet of sebum can sometimes be expressed with pressure. They are usually multiple and lack the arborizing vessels seen in basal cell carcinoma. Cosmetic destructive therapies can be pursued.
Epidermal inclusion cysts
Epidermal inclusion cysts (epidermoid cyst, cyst of the follicular infundibulum, sebaceous cyst) are derived of a wall of keratinizing epithelium depositing keratinocytes into a central cavity. They are clinically recognized as dermal or subcutaneous mobile nodules that are usually asymptomatic and do not require treatment. A central pore is sometimes seen, from which the cheesy, foul-smelling contents can be expressed. Manipulation and trauma sometimes lead to rupture of the wall and subsequent inflammatory response. Incision and drainage typically leads to recurrence, thus an excision including the entire wall of the cyst is a more effective method to prevent recurrence. Episodes of acute inflammation can be treated with incision and drainage or intralesional triamcinolone for temporary relief or with antibiotics if superinfection is strongly suspected.[44]
Malignant skin lesions
Skin cancers are the most common malignancies encountered in the United States and are estimated to affect one in five Americans at some point in their lives. Nonmelanoma skin cancer is not required to be reported to cancer registries so incidence estimates vary, but an estimate based on census data and insurance claims in 2006 suggested an incidence of 3.5 million annually with a cost of over $1.5 billion to the medical system. The majority of these will be in the geriatric population.[1, 45–47] Basal cell and squamous cell carcinomas, referred to generally as nonmelanoma skin cancers, make up the large majority of the skin cancers diagnosed. Most are due to sun exposure and are easily treated. Melanoma makes up a smaller percent of skin cancers but leads to the bulk of skin-cancer-related mortality. All types of skin cancers have shown trends toward increasing frequency over the last three decades, likely due to an increasingly aged population. Although strong evidence supporting routine screening skin exams is lacking, it is generally recommended every one to three years after the age of 40, depending on such risk factors as fair skin, family history, and history of UV exposure. For patients at low risk, this can be performed by a primary care physician who is comfortable providing full skin examinations. Patients with a history of skin cancer, multiple atypical pigmented lesions, >50 moles, or long-term immunosuppression should have an ongoing relationship with a dermatologist for routine monitoring.[1, 46] Increasing evidence supports the use of sunscreen and sun avoidance to prevent the incidence of melanoma and nonmelanoma skin cancers, including in patients who have a prior history of skin cancer.[48–50]
Basal cell carcinoma
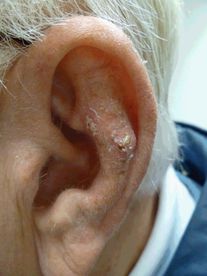
Of the millions of nonmelanoma skin cancers that develop yearly, 75%–80% of these will be basal cell carcinoma (BCC). Fortunately, BCC has very little metastatic potential and, other than in rare cases, is limited to the skin. Men are affected at a rate of 1.5–2:1 when compared to women, and 75%–80% of BCCs will be found on the head and neck.[45–47] The progenitor cell is likely located in the infundibular section of the pilosebaceous unit; thus BCC does not develop on the palms or soles where pilosebaceous units are absent.[48] The median age of development is 68 years. UVR is the main contributor to the development of BCC, specifically via mutations in the gene patched (PTCH1).[51] There is good data demonstrating that regular use of sunscreen and avoidance of tanning (by tanning beds or natural UV exposure) can reduce the lifetime risk of developing this malignancy. Lightly colored hair and eyes, freckles, fair skin, immunosuppression, history of sunburns, radiation exposure, and family history can all contribute to increased risk.[1, 46]
The classic appearance of basal cell carcinoma is a pearly papule or small plaque with arborizing telangiectasias and a rolled border (Figure 35.3). Many subtypes have been described, with the most common being superficial, nodular, and morpheaform (infiltrative, characterized by scarlike features). Ulceration is a finding that is frequently shared among subtypes. Up to 10% of BCCs may have pigment, which may lead to confusion with melanoma. Although mortality is highly unusual, morbidity is frequent. Some BCCs will remain superficial and slow-growing over the course of years, while some will take on aggressive growth patterns and invade into the deep dermis, subcutaneous fat, muscle, or even cartilage and bone. This is especially true on high-risk locations (such as the nose, eyelids, and ears). A small shave biopsy is typically sufficient for diagnosis.[52]
Basal cell carcinoma: pearly papule in the retro-auricular area with arborizing telangiectasias.
Given the high frequency with which BCC is encountered, it is not surprising that many forms of treatment have been developed. Superficial basal cell carcinoma is contiguous with the epidermis and most easily treated. Electrodessication and curettage allows for rapid treatment in the outpatient setting, little discomfort, and high cure rates. Topical therapy with imiquimod or 5-fluoruracil cream has lower cure rates, but is relatively easy to use unless the patient lacks the physical or mental capacity to apply it regularly. Nodular basal cell carcinoma is treated very effectively with electrodessication and curettage but is less successfully treated with topical therapy in most instances. Standard excisions with 4-mm margins and local anesthesia are reliable in the outpatient setting for most types of basal cell carcinoma and is the treatment of choice for more aggressive subtypes when it is feasible.
For high-risk or cosmetically sensitive locations, tumors with ill-defined borders, recurrent tumors, or tumors >2 cm, Mohs surgery is the ideal method of treatment. Mohs surgeons use frozen section pathology to process tissue and read results within about one hour, and take additional layers of tissue until all margins are clear. Most centers achieve recurrence rates of <1%, and the surgery allows for complex closures to take place on the same day the tumor is excised. See Table 35.2 for a summary of common treatments for nonmelanoma skin cancers.[52–54]
Actinic keratosis and squamous cell carcinoma
Actinic keratoses (AKs) are premalignant precursors to squamous cell carcinoma that develop in sun-damaged skin. Although most will remain in a superficial, precancerous stage, there is an estimated rate of 1% chance per decade for each to evolve to invasive squamous cell carcinoma. Since most patients present with many AKs, the risk quickly accumulates and makes treatment of this early stage essential.
AKs are often described to be “better felt than seen,” as they are often subtle visually. They present as erythematous macules or thin papules with sandpaper-like, gritty scale. Palpating high-risk areas such as the forehead, temples, cheeks, and nose is critical for detection. Treating individual lesions with cryotherapy and other destructive techniques is very successful in the office (grade A). Prescription topical treatment is also highly effective for individual lesions or “field treatments” of widespread actinic damage over larger areas.[52]
In many instances, AKs evolve along a continuum to squamous cell carcinoma in-situ (SCCis), which can then progress to invasive squamous cell carcinoma (SCC). Just as often, however, SCCis and SCC will develop de novo. Like AK, SCCis is limited to the epidermis, but the pathologic features fill the epidermis more fully. The clinical distinction can be difficult, but SCCis tends to be larger and is often resistant to superficial treatment such as cryotherapy that is typically very successful in the treatment of AK.[46, 55] Invasive SCC (Figure 35.4) can also be difficult to distinguish from its more superficial counterparts, but it should be highly suspected of lesions that develop thicker, nodular components, have rapid growth, bleed frequently, or are tender to palpation. At times, invasion of local nerves can cause persistent pain or paresthesia. Any lesion that is present or grows for more than six to eight weeks, recurrently ulcerates or bleeds, and does not heal as expected should be biopsied to rule out nonmelanoma skin cancer. As with BCC, shave biopsy is usually sufficient.
Squamous cell carcinoma: crusted nodule on the antihelix, a high-risk location.
A specific subtype of SCC known as keratoacanthoma typically arises and grows quickly, often reaching sizes of 2 cm or greater over the course of four to six weeks. It tends to have a characteristic central crater that is filled with keratinized debris. In some cases this represents a reactive process, and spontaneous resolution over the course of months is not infrequent. Because it cannot be fully distinguished from well-differentiated invasive SCC, most authors recommend surgical removal following diagnosis.[57]
Although SCCis does not have metastatic potential, invasive SCC can become very aggressive in some instances. Estimates of metastatic disease developing in cutaneous SCC vary, but it is likely in the range of 3%–5%. The lips, ears, genitals, and fingers carry an increased risk. SCCis can be treated similarly to superficial or nodular BCC in most instances, whereas invasive SCC should be treated by standard excision or Mohs surgery, depending on the size and location.[46, 56–58] See Table 35.2 for additional information on the treatment of AKs and nonmelanoma skin cancers.
Following the diagnosis of a first skin cancer, the risk of developing another skin cancer over the next three years increases to more than tenfold that of the general population. As more time passes without additional skin cancers developing the risk of subsequent skin cancer gradually falls, but patients remain at increased risk. Although standardized guidelines are lacking, most patients are followed every six months for full skin exams for one to two years following the diagnosis of a nonmelanoma skin cancer and then annually thereafter.[59]
Melanoma
Melanoma makes up less than 5% of skin cancers diagnosed each year, but it accounts for 80% of skin-cancer-related mortality. The rate of melanoma diagnosis is steadily increasing, with an estimated 140,000 melanomas projected to be diagnosed in 2014, which will result in close to 9,700 deaths. Whereas the majority of melanomas are limited to the skin at the time of diagnosis, and survival of those patients approaches 98%, mortality increases significantly in later-stage disease, with <15% of patients surviving at five years after being diagnosed with metastatic disease.[46, 47]
Various factors contribute to the risk of developing melanoma. A number of mutations – including CDKN2A, melanocortin-1 receptor (linked to fair skin and red hair), and BRCA1/2 – are linked to higher rates of melanoma, but environmental risk factors are more common contributors. History of blistering sunburns, cumulative UV exposure, lighter skin types, presence of >50 nevi or atypical nevi, family history, increasing age, and male gender all increase the risk of developing melanoma. Females have higher rates under the age of 40, but the trend reverses, and males over 60 have double the risk of age-matched females.[46, 47]
The clinical diagnosis of melanoma is one of the most challenging aspects of dermatology at any age. Benign nevi (moles) are common benign melanocytic neoplasms that often have overlapping clinical features with melanoma, and 50% of melanomas will develop within nevi. Provider familiarity with the ABCDEs of melanoma (Asymmetry, Border irregularity, multiple Colors, Diameter >6 mm, and Evolution) is helpful, but most nevi with one or two of these features, known as atypical nevi, are benign. When patients have many atypical nevi, it is useful to recognize one or two patterns of “signature nevi” in a patient that have a similar appearance so that a lesion that does not match the background pattern will stand out; this is sometimes referred to as the “ugly duckling” rule.[62]
The primary subtypes of melanoma include superficial spreading, nodular, lentigo maligna, and acral lentiginous. Up to 10% of all subtypes may be amelanotic or hypomelanotic. The superficial spreading subtype (Figure 35.5) is the most common subtype at any age, but lentigo maligna becomes increasingly common in elderly, highly sun-damaged skin. It is named for its resemblance to benign lentigines, and distinguishing features are most often the large size and irregular pigmentation of lentigo maligna. Their subtle and prolonged horizontal growth phase often makes detection difficult, but also results in a long lag time before an invasive component develops. Nodular melanoma is less common but has an early vertical growth phase and often presents at more advanced stages. Acral lentiginous melanoma presents on the palms, soles, and nail apparatus. It is the least common type of melanoma overall, but the most common type found in African-American patients.[1, 63]
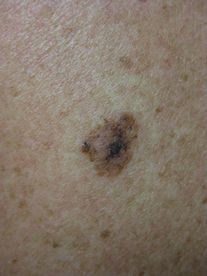
Superficial spreading melanoma: irregularly shaped, pigmented plaque with variegated pigment.
A biopsy should be performed urgently any time melanoma is suspected. Ideally, excisional biopsy with a scalpel or punch tool is utilized to allow for adequate assessment of the margins and depth of the tumor (grade A). The Breslow depth, measured from the stratum granulosum to the deepest point of invasion, is the most important prognostic factor from biopsy specimens, as it is strongly linked to risk of mortality. Melanoma in-situ, which is limited to the epidermis, carries little to no risk of systemic spread. When ulceration is absent and Breslow depth is <1 mm, 10-year mortality risk is <5% in most instances, whereas tumors >4-mm depth with ulceration have 10-year survival of ~ 50%. Advanced age is associated with more advanced disease at the time of diagnosis and poorer prognosis.[1, 46, 63]
Upon diagnosis of melanoma, excision with margins that increase based on the depth of invasion is indicated (grade A). In most tumors <1 mm without ulceration, local excision is curative and no further workup is indicated. A growing role for Mohs surgery in cosmetically sensitive areas for thin melanomas is being seen as data has generally confirmed high clearance rates and excellent cosmetic and functional outcomes.[64] In tumors with >1 mm Breslow depth, a sentinel lymph node biopsy is generally indicated to provide further prognostic information (grade A). Lymph node dissection is typically performed when a sentinel node is positive, but the benefit is controversial, and it carries risk of substantial morbidity.[65–68]
Metastatic melanoma has a poor prognosis, but, after decades of stagnation, major breakthroughs in treatment have emerged. Standard chemotherapeutic regimens have historically had limited success, but as our knowledge of the immune response to malignancy and the oncogenic pathways that fuel melanoma has grown, so has our ability to manipulate these pathways through the use of very specific drugs. Immunotherapy with new biologics – including ipilimumab that inhibits CTLA4 and PD-1 inhibitors – are currently in trials and have shown durable responses in a substantial subset of patients.[69] Targeted therapy with BRAF inhibitors in patients with melanomas harboring BRAF mutations have also led to dramatic responses, although recurrence over several months is seen in >90% of patients.[70] The success of these drugs has been groundbreaking, and it has fueled the ongoing development of many complementary and new agents that are currently in the pipeline.
Similar to nonmelanoma skin cancer surveillance, there are not clear evidence-based guidelines to follow for melanoma screening. Second melanomas develop in 3.5%–4.5% of patients, and local recurrence will occur in more than 4% of patients with the highest risk occurring in the first five years following diagnosis. In most instances, follow-up visits after the diagnosis of melanoma occur every three months for the first year, then every six months in years two through five, and then annually in perpetuity. Visits should include complete skin examination with focus on sites of previous melanoma, lymph node exam, and complete review of systems.[63]
Inflammatory skin diseases
Rosacea
Rosacea is a common, chronic inflammatory condition that predominantly involves the convex surfaces of the central face. It is most common in fair skinned individuals and affects an estimated 1%–10% of this population, with a female predominance.[71–73] Eighty percent of cases will present in adults over 30 years old.[74] There are four morphological subtypes: erythematotelangiectatic (ETR), papulopustular (PPR), phymatous (PhR), and ocular rosacea.[75] It is unknown if the rosacea subtypes represent different stages of a progressive disease, or if they are distinct entities that share common phenotypic features.
Erythematotelangiectatic rosacea is characterized by persistent centrofacial erythema, facial flushing, and scattered telangiectasia on the forehead, nose, and malar cheeks. Flushing is often the primary initial and ongoing symptom of cutaneous rosacea, and it can be accompanied by symptoms of stinging or burning. Exacerbations are often triggered by alcohol, ultraviolet radiation, caffeine, heat, emotion, smoking, and spicy foods. These factors are thought to induce rosacea by dilation of cutaneous vessels.[71] As the name implies, papulopustular rosacea is defined by the development of papules and pustules that typically involve the same distribution in the central face. Lesions can appear acneiform, but unlike acne vulgaris, there are no comedones (Figure 35.6). Phymatous rosacea is easily recognized by marked, nodular tissue hypertrophy that distorts the facial contours. The nose is most commonly involved (rhinophyma), but the chin, cheeks, and ears can also be affected. The fourth subtype, ocular rosacea, must be considered in all patients with cutaneous rosacea. Ocular symptoms are variable and have a range of severity from chronic hyperemia, conjunctivitis, and blepharitis to corneal ulceration and vision-threatening keratitis. Symptoms include redness, burning, watering, dryness, and light sensitivity. Ocular disease has been reported to be present in 33% of patients with skin manifestations and does not necessarily reflect the severity of the skin disease.[76] Eye involvement may precede, occur concurrently, or follow cutaneous disease. It may also occur in the absence of cutaneous disease. Any patient with signs or symptoms of ocular rosacea should be referred for an ophthalmologic evaluation.
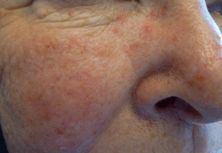
Rosacea: erythema, telangiectasias, and scattered papules and pustules.
Although the precise etiology of rosacea is unclear, research continues to implicate the innate immune system in the pathogenesis of the disease. In affected individuals, exposure to environmental triggers such as ultraviolet light and commensal skin organisms results in an inappropriate activation of the innate immune system. This aberrant activation results in the inflammation and neurovascular hyperreactivity that define the disease. Although there may be a genetic predisposition to developing rosacea, no specific genes have been implicated. The role of the saprophytic mite, Demodex folliculorum, is unclear, although mite proteins have been shown to induce innate inflammatory responses.[77] Higher mite density has been found on people with rosacea, and decreasing the mite density is associated with reduced inflammation in recent studies.[78]
All patients with rosacea should be counseled to avoid triggers such as temperature extremes and alcohol, practice sun protection, and use only gentle, hypoallergenic skin products. Cosmetic camouflage with green-tinted facial powder or foundation may be recommended.
Medications classically utilized for papulopustular rosacea include topical metronidazole, azelaic acid, and sulfacetamide-sulfur formulations. For patients who fail topical therapy, either subantimicrobial or fully bacteriocidal doses of oral tetracyclines are the next line therapy. The anti-inflammatory component of tetracyclines, in particular the ability to inhibit proteases, is thought to be responsible for their efficacy. For patients with ETR or persistent erythema, topical brimonidine is a new agent that is available. It is an alpha-2 adrenergic receptor agonist and works immediately to vasoconstrict dilated vessels and diminish redness. Side effects include irritation and risk of rebound erythema and should be discussed with the patient. Topical retinoids are also utilized and are thought to work by inhibiting inappropriate innate immunity activation. Pulsed dye or potassium titanyl phosphate (KTP) lasers are efficacious treatment for both persistent erythema and telangiectasias. They target hemoglobin, resulting in heating and coagulation of the vessels. In severe cases of inflammatory PPR or early phymatous rosacea, oral retinoids such as acitretin or isotretinoin may be beneficial. Adjunctive therapy for phymatous rosacea includes surgical debulking with dermabrasion or laser ablation with a carbon dioxide laser to re-contour the hypertrophic tissue. In all cases, neither oral nor topical steroids are recommended because of the likelihood of inducing dependence and a rebound steroid-induced rosacea.[1, 6, 7, 71]
Seborrheic dermatitis
Seborrheic dermatitis is a chronic inflammatory condition that has a characteristic clinical appearance and distribution. It is recognized as chronic, relapsing erythema that involves the glabella, eyebrows, paranasal region, alar creases, and nasolabial folds. The chest, frontal hairline, and ear canals are also frequently involved (Figure 35.7). There is also a variable degree of skin flaking, from fine dandruff (pityriasis sicca) to thick, greasy adherent scales (pityriasis steatoides).
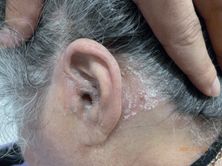
Seborrheic dermatitis: erythematous plaques that are flaky, adherent, and slightly greasy affecting the scalp, ear, and retroauricular sulcus.
Although seborrheic dermatitis has a predilection for areas of the body with increased numbers of sebaceous glands, it is not felt to be a disease of sebaceous glands. The etiology is thought to be due to the host response to commensal saprophyte Malassezia (formerly Pityrosporum ovale).[1, 6, 7, 79] The fungus is not believed to be directly pathogenic; rather, it may incite a host immune response or cause an irritant dermatitis from fungal metabolic byproducts. Patients with Parkinson’s disease or HIV may have severe seborrheic dermatitis. The reason for this is unknown, but it improves with treatment of both conditions.
Seborrheic dermatitis can be mild without symptoms or can be quite impressive with erythema and thick scaling (pityriasis amiantacia) of the scalp. There may be itching and, rarely, alopecia. Mild disease is usually controlled with topical antifungals such as ketoconazole 2%, selenium sulfide 2.5%, or ciclopirox 1% shampoo. The shampoo may be used for scalp and/or skin involvement, and the patient is asked to leave the medication on for five to ten minutes before washing it off. It is usually used two to three times a week until control is established, after which it may be used weekly for maintenance. Topical antifungal creams such as ketoconazole 2%, other azole creams, and ciclopirox 1% cream may also be applied directly if preferred. They are also used daily until symptoms are controlled, then decreased to weekly for maintenance. For inflammatory seborrheic dermatitis, topical steroids are beneficial. The strength and formulation chosen are dependent on the location being treated. For the face, low-potency steroids, such as hydrocortisone 2.5% or desonide 0.05% cream, should be used twice a day until improvement. For the scalp and body, mid- to high-potency steroids can be safely used. For the scalp, fluocinolone acetonide 0.01% shampoo is approved by the US Food and Drug Administration, although other formulations (such as solutions or foams) are frequently used off-label, depending on the patient’s vehicle preference. Careful consideration must be made if there is a component of rosacea or periorificial dermatitis, because topical corticosteroids are known to cause flares in both of these conditions. Occasionally, short courses or intermittent suppressive doses of oral itraconazole or fluconazole can be beneficial for flares or severe disease.[79]
Whenever seborrheic dermatitis is resistant to therapy, alternative diagnoses should be considered. The primary differential diagnosis of seborrheic dermatitis includes rosacea, psoriasis (particularly for scalp involvement), tinea corporis, systemic lupus erythematosus (SLE), and periorificial dermatitis. History and physical exam are usually sufficient for differentiating these entities.
Psoriasis
Psoriasis is an immune-mediated inflammatory disorder that can have a variety of clinical presentations and can vary greatly in severity. It is most commonly characterized by erythematous papules and plaques with silvery scale (Figure 35.8) and has several subtypes, including plaque, guttate, inverse, erythrodermic, pustular, and nail psoriasis. It affects 1%–3% of the world’s population, and 3.2% of all psoriatic patients have an onset in the geriatric age group over 60 years old.[80] Diagnosis is usually clinical, but a skin biopsy may be required in nonclassical presentations. It is important to approach psoriasis as a systemic disease. It is associated with systemwide inflammation that has deleterious effects on the cardiovascular and hepatic systems. Psoriasis has been shown to be independently associated with an elevated risk of coronary artery disease, type 2 diabetes mellitus,[81] and nonalcoholic fatty liver disease in patients over 55 years old.[82] Other comorbidities that are associated with psoriasis include malignancy, diabetes, metabolic syndrome, depression, inflammatory bowel disease, and serious infections. Psoriatic arthritis (PsA) has been reported to affect from 5% to as high as 30% of patients with psoriasis,[83] and there is a yearly incidence of newly diagnosed PsA of almost 2% per year.[84, 85] A rheumatologist should evaluate patients with suspected joint involvement.
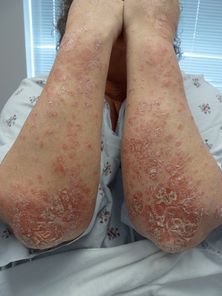
Psoriasis: scaly, erythematous plaques with a predilection for extensor surfaces.
When evaluating the patient with psoriasis, it is important to consider potential triggers such as infection or medications. Streptococcus pyogenes has been associated with new onset of guttate psoriasis as well as flares of existing disease. Medications may also trigger or exacerbate psoriasis. The worst offenders include antimalarial drugs, beta blockers, and lithium. Angiotensin-converting enzyme inhibitors, NSAIDs, and terbinafine have also been reported as inciting agents.[86] Abrupt cessation of corticosteroids is known to provoke erythrodermic and pustular flares, and the TNF-α antagonists have triggered palmoplantar and diffuse pustular psoriasis in patients without a prior history of the disease.
The treatment of psoriasis can be approached in a stepwise manner, and the chosen agents and formulations should be tailored to patient comorbidities as well as preference. The first step in treatment is topical therapy, which includes medium- to high-potency topical steroids (grade A), vitamin D analogs such as calcipotriene and calcitriol (grade B), and tar-based emollients (grade B). For facial or intertriginous areas, low- to mid-potency steroids, calcipotriene, or topical calcineurin inhibitors (such as tacrolimus or pimecrolimus) may be used as alternatives (grade B). For patients who do not have optimal response to topical therapy but are not candidates for systemic therapy, light therapy with nbUVB (311 nm) is a useful adjunct. First-line oral therapies for psoriasis include methotrexate and acitretin (grade A). In severe cases of acute pustular or erythrodermic psoriasis, cyclosporine or infliximab should be instituted due to the rapid onset of action (grade C). For long-term maintenance, methotrexate at 7.5 mg–25 mg weekly is a generally well-tolerated medication that can be tailored to a patient’s glomerular filtration rate (GFR). Laboratory monitoring is required, and cumulative methotrexate dose should be followed to determine the need for liver biopsy. Acitretin may be used in the absence of severe renal insufficiency, but it is often harder to tolerate. Side effects include dry skin, photosensitivity, and arthralgia. Acitretin is particularly effective in the treatment of localized palmoplantar pustular psoriasis and is often a first-line agent in such cases. As with methotrexate, laboratory monitoring is required. The biological drugs – including the TNF-α inhibitors (adalimumab, etanercept, and infliximab) and the anti-IL-17/23 monoclonal antibody, ustekinumab – are also options in the elderly population (grade B). The biologics are associated with a small risk of infection, and the patient should be evaluated for prior exposure to tuberculosis, hepatitis B, and endemic fungal infections prior to initiation. The TNF-α blocking agents have also been associated with demyelinating diseases and hematologic malignancies; however, it is not clear whether the increased risk is due to the underlying disease state or the medication. There is no convincing evidence that relative risk of infection with TNF-α therapy increases with age.[86] Lastly, there are new oral medications on the horizon for the treatment of psoriasis. The janus kinase (JAK) inhibitors have been approved for rheumatoid arthritis, and these – along with phosphodiesterase 4 inhibitors and Th17 pathway inhibitors – are in the final stages of the FDA approval process. Preliminary studies are showing excellent tolerability as well as efficacy.
Atopic dermatitis
Atopic dermatitis (AD) is a common, chronic inflammatory condition of the skin that is estimated to have a lifetime prevalence of about 20%.[87] AD usually has an onset in infancy to early childhood. Adults with AD usually developed the condition as a child; however, there is also a small subset of individuals who present in adulthood. Most studies report 20- to 40-year-olds as the most common age of adult-onset AD, but others have reported an age of onset in patients as old as 79.[88] AD is characterized by erythematous, scaly plaques that range in severity and distribution. In adults, the hands, feet, and the flexural surfaces are frequently involved. Less common, nonflexural morphological variants also occur and include nummular (Figure 35.9), follicular, seborrheic dermatitis-like, and prurigo-like patterns.[88] Affected areas are pruritic, and constant rubbing and inflammation may lead to lichenification of the skin. Complications of AD are often infectious in nature and include bacterial superinfection, dermatophytosis, and eczema herpeticum.
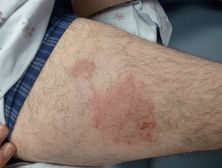
Nummular dermatitis: annular, slightly scaly, with pruritic plaques on the extremities.
Diagnosis is usually made clinically, although a biopsy may assist in ruling out other diagnoses. Pruritus is always present and can be helpful in distinguishing AD from other common conditions such as psoriasis. Assessing for an allergic contact dermatitis with patch testing is often performed in new adult-onset disease or in cases where there is acute worsening. One study showed nearly one-third of 1,022 patients with nummular dermatitis had a reaction to one or more allergens (nickel sulfate was highest at 10.2%).[89] Other conditions on the differential diagnosis that should be considered include pityriasis rosea, lichen simplex chronicus, scabies, tinea corporis, and seborrheic dermatitis.
The etiology of atopic dermatitis is multifactorial. The discovery of the filaggrin mutation in some patients indicates that at least a subset is caused by skin barrier dysfunction. Other subtypes may be predominately due to abnormalities in the immune system. Both of these ultimately result in abnormalities in cutaneous immunity, which is critical for protection against pathogens as well as generating tolerance to benign antigens. These effects account for the clinical findings and complications of AD.
Treatment options range with the severity of disease and degree of impact on the quality of life. All patients should be asked to maintain skin hydration and avoid trigger factors such as heat, low humidity, and any contact allergens that have been identified. Avoidance of aeroallergens and food allergens, although reasonable, has not been shown to be useful for disease control.[90–92] Patients with AD should also be evaluated for superinfections, because they are at an increased risk of bacterial, viral, and fungal skin infections. The mainstay for mild disease is mid- to high-potency topical steroid preparations on the trunk and extremities, and low- to medium-potency topical steroids on the face (grade A). The use of high-potency ointments is frequently employed by dermatologists who prefer short bursts of high-potency steroids to longer treatments with mild- to medium-potency steroids. Aggressive moisturization with thick cream or emollients is important for preserving as much barrier function as possible. Antihistamines can be a useful adjunct for the associated pruritus, particularly for nocturnal itching. For disease that is recalcitrant to topical regimens, narrowband UVB (311 nm) or oral immunomodulating medications are warranted. First-line therapies include methotrexate, azathioprine, and mycophenolate mofetil (grade B). Each has variable efficacy amongst patients, and the first agent is often chosen based on patient comorbidities and the drug side effect profiles. In the case of acute flares, cyclosporine 5 mg/mL/day may be used to establish quick control prior to transitioning to a long-term immunomodulator (grade B).[93]
Pruritus
Pruritus is a common complaint in the elderly population that can be debilitating for patients and challenging to treat. Each year in the United States, more than seven million patients complain of itching at outpatient visits; of these, 1.8 million (25%) are complaints from patients 65 years or older.[94] It is often very frustrating for the patient and can significantly impact the quality of life, causing sleep disturbances and depression. The approach to pruritus involves a thorough history and physical examination and careful exclusion of an infectious etiology, medication side effect, or systemic disease. A complete dermatologic examination should be performed to look for primary or secondary skin lesions.
Pruritus can be grouped into pruritus with skin disease, pruritus without skin disease, and pruritus resulting from secondary skin changes, usually from chronic rubbing or scratching (Table 35.3). If there is skin disease, diagnosis and treatment should be directed at the eruption. This involves careful evaluation of morphological features, distribution, symptoms, and occasionally a skin biopsy. Principal subgroups include primary skin diseases such as atopic dermatitis and Grover’s disease, infectious diseases, medications, and physiologic changes of aging skin. Impaired skin barrier function and immunosenescence are two well-described phenomena that occur in aging skin and result in pruritus. Immunosenescence results in impaired cellular immunity, increased risk of infection, and a cytokine profile that favors inflammation and itch. Skin dryness, or xerosis, worsens over time, and clues that it is causing itch include: temporary relief during bathing, worsening in the winter months, and itching of the extremities more than the trunk. Xerosis should always be treated regardless of whether it is the primary cause of itch. The patient should be asked to avoid soaps, detergents, and astringents, to limit bathing to less than once a day, and to avoid hot water. Application of petrolatum or heavy cream moisturizer should be performed immediately after bathing and throughout the day. Wet wraps may be utilized in severe cases.[95]
CBC – complete blood count, TFT – thyroid function tests, LFT –liver function tests, BUN – blood urea nitrogen, Cr –creatinine, Ca –calcium, Phos – phosphorus, TFTs – thyroid function tests, PTH – parathyroid, CsA – cyclosporine A, MMF – mycophenolate mofetil
Pruritus can also be caused by chronic rubbing or picking of an initial primary lesion or normal skin. The skin’s response is to thicken and form well-demarcated, lichenified plaques or papules that we recognize as lichen simplex chronicus (Figure 35.10) and prurigo or “pickers” nodules. Once established, these changes can perpetuate the “itch-scratch” cycle even if the primary cause has resolved. Treatment can be extraordinarily challenging and involves a discussion of the “itch-scratch cycle” with the patient and possibly a psychiatric evaluation. Systemic medications have been used in severe cases.
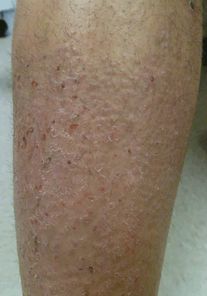
Lichen simplex chronicus: thick, excoriated, lichenified plaque on the lower leg at a site of frequent scratching due to contact dermatitis.
If there is no rash, this usually indicates a systemic or neurologic cause, and the focus should be directed at patient comorbidities and medications (see Table 35.4).
| Agent | Examples* | Applications |
|---|---|---|
| Topical steroids | Desonide | Primary skin diseases excluding infectious |
| Triamcinolone | Secondary skin diseases | |
| Clobetasol | ||
| Topical calcineurin inhibitors | Pimecrolimus | Alternative to steroid for chronic use or for use on face/genital/intertriginous areas |
| Tacrolimus | ||
| Topical cooling agents | Menthol | For soothing effect; can be kept in the refrigerator for added cooling effect |
| Camphor | ||
| Phenol | ||
| Topical anesthetics | Pramoxine 1% cream | Focal pruritus and neuropathic etiologies |
| Eutectic mixture of lidocaine 2.5% + prilocaine 2.5% (EMLA) | ||
| Lidocaine in acid mantle cream | ||
| Lidocaine patch | ||
| Other topicals | Topical Doxepin 5% | Atopic dermatitis |
| Neuropathic itch | ||
| Topical capsaicin 2%–6% | Neuropathic itch | |
| Systemic antihistamines | Diphenhydramine | Focal or systemic pruritus; used frequently despite lack of evidence of efficacy for diseases that are not specifically histamine mediated |
| Cetirizine | Use in geriatrics may be limited by anticholinergic effects and confusion | |
| Fexofenadine | ||
| Loratidine | ||
| Other systemic medications | Gabapentin 100 mg–300 mg and increased up to 1,800 mg/day. | Neuropathic itch |
| Some primary skin disease | ||
| Itch associated with systemic disease | ||
| SNRI, esp. mirtazapine | ||
| SSRI such as paroxetine, sertraline, fluvoxamine | ||
| Doxepin | Nocturnal pruritus | |
| Adjuvant therapy | nbUVB | ESRD |
* Topical steroids are typically applied twice a day to affected areas until improvement or up to two weeks.
SNRIs – selective norepinephrine reuptake inhibitors, SSRIs – selective serotonin reuptake inhibitors
Note: Topical antihistamines generally are not recommended due to the risk of irritant or contact dermatitis and lack of efficacy.
In some patients, no clear cause will be found on thorough workup. For these patients, a combination of age-related skin changes is often the diagnosis of exclusion. Management is then centered on symptom management and identification of triggers or exacerbating agents.
Lower extremity stasis-related skin changes
Lower extremity chronic venous insufficiency is a common finding in the elderly and is often multifactorial in etiology. There are several dermatoses that are related to chronic venous stasis, the most common of which is stasis dermatitis.
Stasis dermatitis affects a reported 6.2% of patients over the age of 65 and results from chronic venous hypertension.[118] The skin overlying the affected areas can be brightly erythematous or violaceous, or it can appear rusty due to red blood cell extravasation and hemosiderin deposition in the dermis. Eczematous patches or plaques with scaling are typical, and in acute cases the skin can be weeping and vesicles may be present. It is not uncommon to have skin breakdown with ulcerations or widespread weeping of serosanguinous transudate.
The treatment for stasis dermatitis is treating the cause of the edema and encouraging fluid redistribution with compression hose, leg elevation, and daily walking. White petroleum jelly is an effective, inexpensive, and non-sensitizing product that may be used to protect and lubricate the skin. Mid- to high-potency corticosteroids such as triamcinolone ointment, with or without occluding leg wraps, are a mainstay of therapy when erythema suggests underlying inflammation. Wet dressings with antiseptic solutions – such as dilute acetic acid, potassium permanganate, or aluminum acetate or subacetate – for two to three hours, two to three times a day followed by application of emollients are useful for weeping or impetiginized areas. In cases of ulceration, barrier creams like zinc oxide are also important to protect the skin from further breakdown and environmental exposures. Zinc oxide is also available in an impregnated bandage that can be wrapped to create compression (Unna boot). It is important to note that patients with stasis dermatitis are prone to developing contact allergies, and common sensitizers such as neomycin and bacitracin should be avoided.
Areas of stasis dermatitis are also prone to infection. Impetiginized lesions can be treated with antiseptic wraps or topical mupirocin, which is least likely to cause cutaneous sensitization.[119, 120] Secondary cellulitis requires oral antibiotic therapy. Of note, cellulitis can appear similar to stasis dermatitis with warmth, erythema, and edema; a key differentiating feature is the distribution. Stasis dermatitis is almost invariably bilateral, and it is exceedingly rare for cellulitis to be bilateral.
There are several other skin diseases that primarily affect the lower legs and are thought to be associated with chronic venous stasis or other vasculopathy. Acroangiodermatitis is characterized by scaly, red to violaceous papules and plaques located circumferentially on the lower extremities. This condition is characterized by arterio-venous shunts that develop after hyperplasia of the vasculature due to venous hypertension. Schamberg disease is a pigmented purpuric dermatosis that commonly affects the lower extremities and may be caused or potentiated by edema. It appears as small rusty macules with a “cayenne pepper” appearance. The rusty appearance is due to hemosiderin extravasation and deposition caused by superficial capillaritis. It is usually chronic and asymptomatic. Other lower extremity vasculopathies include vasculitis of medium and small vessels, such as leukocytoclastic vasculitis and erythema elevatum diutinum. These vasculitities are often found on the lower extremities, and if they are suspected, the diagnosis is made by skin biopsy. Lastly, lipodermatosclerosis is a lower extremity finding that is related to both diabetes and lower extremity edema. Cutaneous sclerosis with fat degeneration and dermal fibrosis results in hyperpigmented, shiny atrophic appearance of the skin and narrowing of the ankles; often with an “inverted bottle” appearance.
Autoimmune blistering disorders
Autoimmune blistering disorders (ABD) are characterized by the development of autoantibodies against cell-cell adhesion molecules. The distribution and quality of the blisters are defined by the properties of the target antigen. Autoimmune blistering disorders may be broadly divided into pemphigus and pemphigoid. Pemphigus antigens are located within the epidermis, and the blisters are shallow and clinically flaccid. Pemphigoid antigens are located at the basal layer, and the blisters are therefore deeper and tense when intact (Figure 35.11).[121] Pemphigus and pemphigoid each have subtypes that are clinically distinct; they affect different skin surfaces, have different responses to therapy, and have different natural courses. (See Table 35.5 for a brief review of these disorders.)
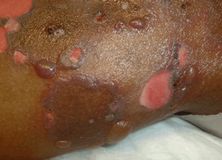
Bullous pemphigoid: widespread, tense bullae erupting diffusely on the trunk and extremities. Shallow ulcers are present where bullae have ruptured.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree