Fig. 28.1
a, b 45-year-old patent had a skin-sparing mastectomy and SLNB with a D-IBR using a tissue expander. c Five months after the primary operation, a textured, anatomic shaped 600 cm3 silicone implant was placed to the right side and a textured, round, moderate plus profile 200 cm3 implant with a mastopexy was performed for symmetrisation on the left side. d, e Additionally the reconstruction of the nipple and tattooing was completed
According to Kronowitz the decision of when to perform BR remains controversial and will often depend on individual circumstances in addition to the need for adjuvant RT [9]. In planning BR, effective oncological treatment is considered the top priority, and the aesthetic goals of reconstruction are subordinate to this [3].
28.2 Indications and Special Considerations for Delayed Breast Reconstruction
Breast reconstruction may be an option for any breast cancer patient undergoing surgery [1, 5, 14] and who is physically and mentally suitable without compromising definitive oncological therapy or likely to be at high risk of surgical morbidity or mortality [14]. Even stage IV disease is not a contraindication for BR if the patient’s predicted life expectancy is relatively long, and surgery will not delay or prevent life-prolonging systemic treatments. MDT involvement in such cases is mandatory [5]. Patients should be provided with appropriate sources of both written and verbal information, detailing the risks and benefits of different types of BR [14]. The assessment should take into account all of the oncological and reconstructive factors, in light of the individual circumstances and preferences of each patient, irrespective of whether the optimal reconstructive method is available locally or not [14]. Oncological principles must not be compromised and should always be prioritized [14].
When DBR is considered, the results of a full clinical assessment and staging should be available for assessment [1]. Maintaining close communication between plastic or oncoplastic surgeons and other team members is essential [14]. For each patient a plan of the reconstructive procedure must be drawn up. The plan defines the expected staged (multiple-step) approach, the risk of morbidity, estimated operation time, length of hospital stay and rehabilitation therapies [14]. MDT members should agree on the offered DBR options, and the patient should be fully involved in the decision-making process [5, 14].
28.2.1 Oncological Considerations for Delayed-Immediate and Delayed Breast Reconstruction
If DBR is considered, full clinical assessment and staging are mandatory [14]. Preoperative unrecognized locoregional recurrences can result in major difficulties, for example, if there is a need to perform an axillary lymphadenectomy shortly after microsurgery in the axilla. The first step of DBR is complete excision of the scar tissue [9]. Tissue excised from the former cancer site should be sent for histopathology. If the tissue is suspicious for malignancy, it should be investigated intraoperatively by frozen section before proceeding, and if a recurrence is identified, the tumour must be removed radically, and BR may need to be delayed and replaced by salvage surgery which may require use of flaps (see ► Chap. 22, Surgery for Recurrent Disease).
For D-IBR, the probability of adjuvant treatment (especially RT) is an important factor in decision-making [14]. RT may exert a harmful effect on the reconstructed breast particularly following implant-based procedures [1, 14, 15]. The metal ports of some tissue expanders may interfere with RT dosage and dose distribution [14]. The surgery to exchange the expander to the permanent implant may be performed prior to or after completion of the RT; however, expander to implant change prior to RT is associated with a higher rate of capsular contracture, malposition, poor cosmesis and implant exposure [9, 15] (◘ Fig. 28.2, ◘ Table 28.1).
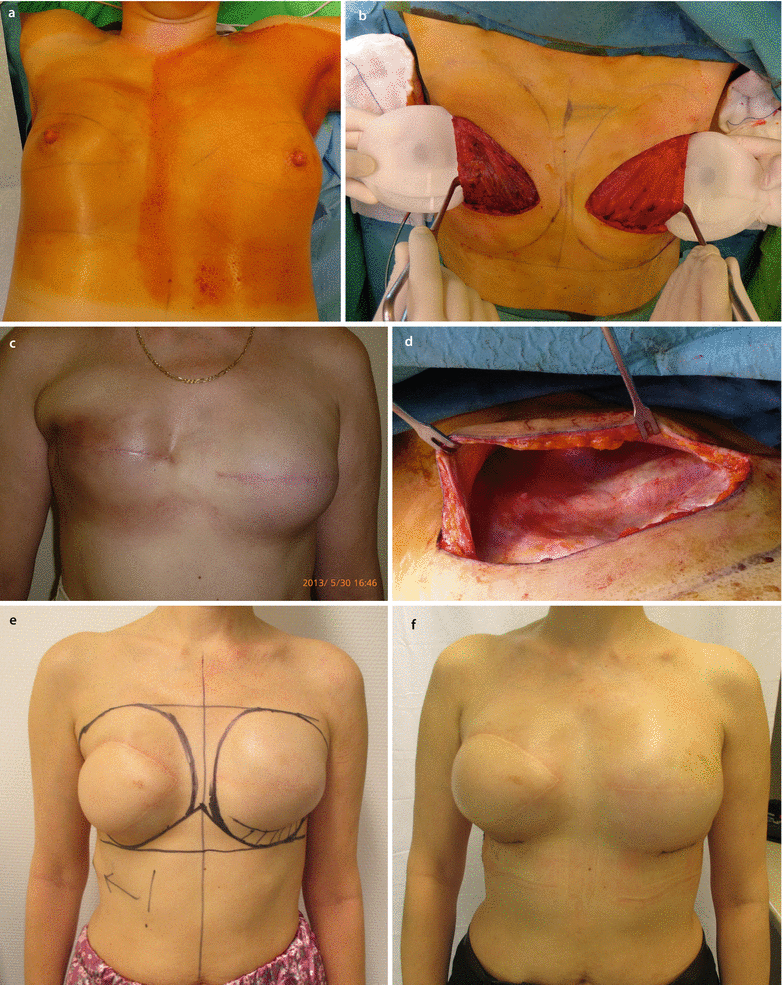
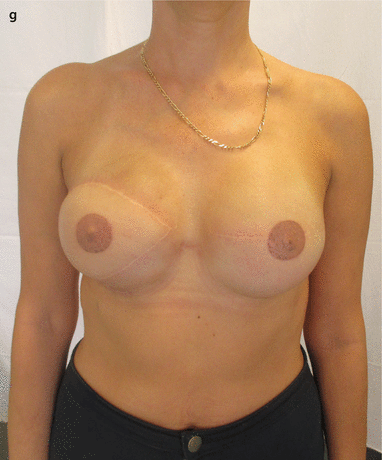
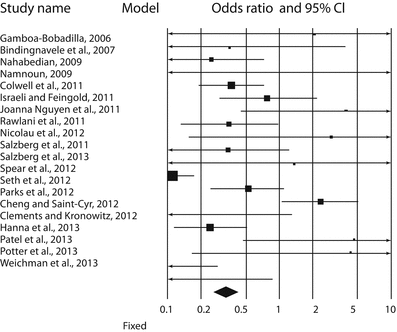


Fig. 28.2
a 51-year-old patient had a right BCS and SLNB in 2011 and subsequent radiotherapy. BRCA2 mutation subsequently identified. In 2013 a second primary tumour in the left breast was diagnosed. b SSM of the right side and SSM and SLNB on the left side and D-IBR using tissue expanders were performed. On the right side the differences in colour, texture and elasticity of the former irradiated major pectoral muscle can be seen. c, d After the partial expansion on the right side, a Baker IV capsular contracture occurred, causing the impression of the thoracic wall. e Six months later the fibrotic breast skin remnant was excised, the expander was explored and removed and the soft tissue was reconstructed with an LDmc flap and placement of a tissue expander. f Three months later a symmetrization was done by using a textured anatomic-shaped 545 cm3 silicone implant on both sides. g Nipple reconstruction and tattooing were performed
Table 28.1
Forest plot of 20 studies by Valdatta et al. [16]. The authors reported the complications occurring in ADM-assisted immediate implant breast reconstruction, with or without radiotherapy. Odds ratios and confidence intervals at 95% are plotted. The black diamond at the bottom is the pooled odds ratio, and it is CI 95%. It completely falls to the left of 1.0 [16]

The timing of DBR, or a staged expander to implant exchange in case of a D-IBR, is recommended at the earliest 1–3 months after the completion of the adjuvant chemotherapy or 3–6 months after RT [9]. An important consideration in DBR is that of concern that IBR may result in delayed adjuvant systemic therapy if there are complications; however, data suggests this effect is minimal (◘ Fig. 28.3) [17].
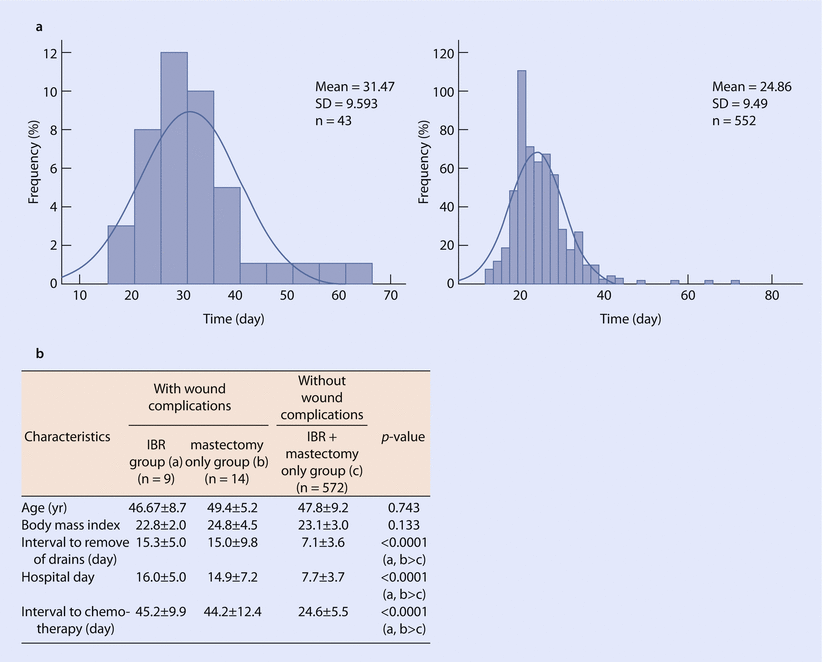

Fig. 28.3
Effect of immediate reconstruction on chemotherapy timing. a Time interval to chemotherapy in women who have or have not undergone chemotherapy. b Comparison of complication rates in women undergoing reconstructive surgery with/without wound complications and the impact on chemotherapy timing (Values are presented as mean ± SD. IBR immediate breast reconstruction, SD standard deviation) (Reproduced from Lee et al. [17] with permission from The Journal of Breast Cancer)
The effect of adjuvant RT following autologous flap reconstruction is controversial [18]. When postmastectomy RT is indicated, autologous tissue reconstruction is either delayed until the end of the RT or D-IBR could be performed followed by flap transposition [18] (◘ Fig. 28.4). Some experienced breast cancer teams have implemented protocols in which IBRs are followed by RT without significantly affecting breast volume after deep inferior epigastric perforator (DIEP) flap reconstruction [18]. Women requiring postoperative RT should not be discouraged from undergoing immediate DIEP flap reconstruction, but RT is generally preferred to precede the flap transfer, because of the reported decreased aesthetic end result [18].
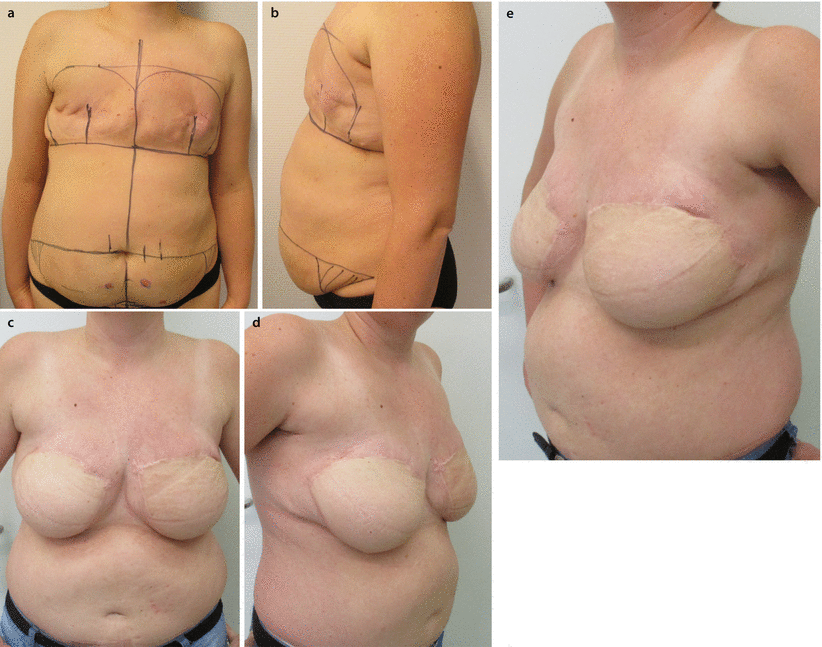

Fig. 28.4
a, b The 38-year-old patient was operated in 2007 on the right breast with BCS and SLNB and adjuvant RT. In 2008 she had cancer on the left side treated with BCS and SLNB and RT. BRCA testing was negative. In 2013 bilateral mastectomy and reSLNB on the right side was performed due to a rpT1cpN0(sn) recurrent NOS cancer c–e. In 2014 bilateral muscle-sparing TRAM reconstruction was performed after flap delay
Tissue expansion of previously irradiated skin can result in a significantly increased risk of capsular contracture, implant malposition, poor cosmesis, implant exposure and failed BR and is therefore relatively contraindicated [9, 18]. In these cases autologous tissue reconstruction is the preferred method [18].
There is some controversy to whether autologous IBR with adjuvant RT is associated with acceptable complication and cosmetic outcomes. The meta-analysis of Schaverien and colleagues (no randomized controlled trials met the inclusion criteria only observational studies were analysed) regarding outcomes of autologous IBR with postoperative RT compared with no RT, as well as with autologous DBR following postmastectomy RT, revealed no significant differences in total prevalence of complications or revisional surgery and a summary measure for fat necrosis favouring the group without RT (OR 2.82, 95% CI 1.35–5.92, p = 0.006) [19]. Most of the studies comparing IBR and postoperative RT with DBR following adjuvant RT reported satisfactory outcomes following IBR. There was no significant difference in overall incidence of complications and fat necrosis (OR 0.63, 95% CI 0.29–1.38, p = 0.25) and a summary measure for revisional surgery (OR 0.15, 95% CI 0.05–0.48, p = 0.001) favouring the DBR group. This meta-analysis reported satisfactory outcomes and a similar incidence of complications for autologous IBR and adjuvant RT when compared with no RT or delayed reconstruction following RT, although the proportion that required revisional surgery was higher for immediate than DBR. The authors highlighted that these findings are limited by the paucity of high-quality data in the published literature, and until better data is available, the findings of this review suggest that autologous IBR should at least be considered when adjuvant chest wall RT is anticipated [19] (◘ Table 28.2).
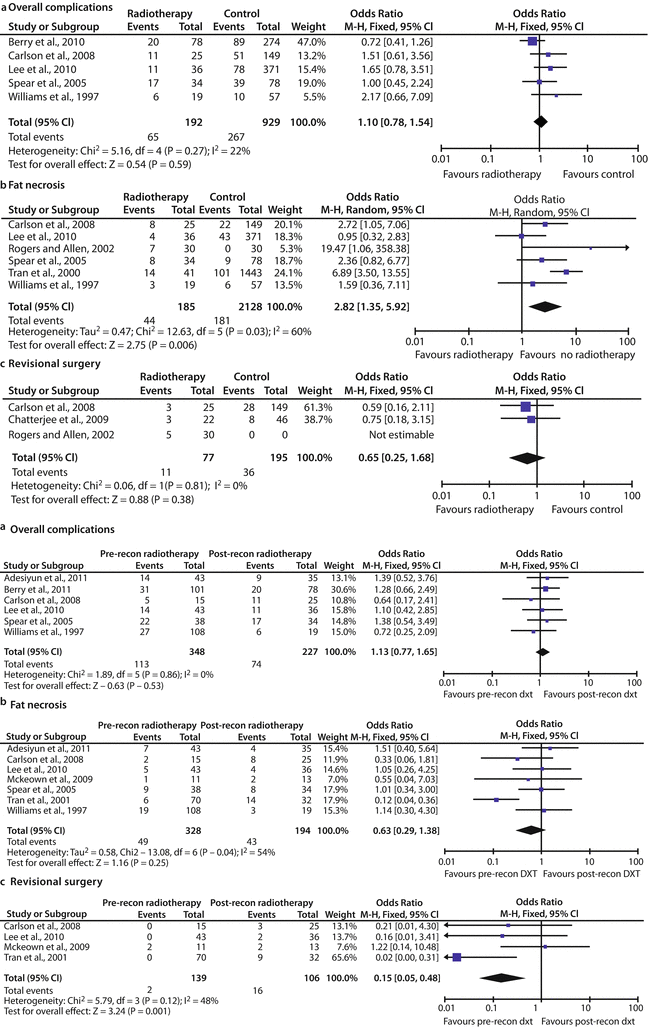
Table 28.2
Complications of autologous breast reconstruction with or without postoperative radiotherapy according to the meta-analysis by Schaverien et al. a Forest plot of prevalence of complications. b Forest plot of prevalence of fat necrosis. c Forest plot of prevalence of revisional surgery

According to the consensus statement of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer in 2015 for patients whose breast cancer was diagnosed during pregnancy, IBR in the first instance and D-IBR with a submuscularly placed tissue expander can be considered [20].
IBR is contraindicated in conjunction with mastectomy for inflammatory breast cancer (IBC) because of the high recurrence rate, aggressive nature of the disease and the need for adjuvant RT without any potential delay [18]. Skin-sparing mastectomy has not yet been proven to be safe in IBC. According to the National Comprehensive Cancer Network (version 2.2016) guidelines, DBR can be recommended to women with IBC who have undergone a modified radical mastectomy [18].
28.2.2 Patient-Related Factors
Patient-related factors play an important role in the timing and choice of reconstructive technique. These factors include performance status, comorbidities, body mass index (BMI), ASA score, drug and smoking history, psychological suitability, occupation, daily activities and lifestyle, pre-existing shoulder or musculoskeletal problems, patients’ expectations and choice, goals, attitudes to risk and the likely impact of recovery time on family [14]. The psychological status of the patient should be assessed preoperatively [14]. If additional risks exist, they should be clearly stated when consenting the patient [14].
According to the current evidence-based clinical practice guideline, breast reconstruction with expanders and implants, of the American Society of Plastic Surgeons, smoking increases the risk of postoperative wound complications and implant loss in patients undergoing postmastectomy expander/implant BR [21]. The overall complication rates were 2.2–3.07 times higher among smokers than non-smokers. Smokers were 2.9 times more likely than non-smokers to develop wound necrosis (p = 0.003) and 5.9 times more likely to experience reconstruction failure (p = 0.001).
Evidence shows that obesity increases the risk of postoperative complications in patients undergoing postmastectomy expander/implant BR [21]. BMI >30 was significantly associated with postoperative wound infections and expander/implant failures. Wound infections among patients with immediate expander/implant reconstructions were 3.3 times higher among patients with a BMI of 25–30 (p = 0.002) and 18.5 times higher among those with a BMI >30 when compared to patients with a BMI <25 (p < 0.001). The risk of implant loss was 3 times higher for those with a BMI of 25–30 (odds ratio 3.1 [95% CI 1.0–9.3]; p = 0.043) and almost 6 times higher for those with a BMI >30 when compared to those with a BMI of <25 (odds ratio 5.9 [95% CI 1.2–29.5]; p = 0.032). Several studies found a statistically significant link between obesity and an increased risk of mastectomy skin flap necrosis, fat necrosis, wound dehiscence, infection, seroma, hematoma and implant extrusion. Obese patients were almost twice as likely as patients of a normal weight to develop an expander/implant complication (odds ratio 1.8 [95% CI 1.1–3.0]; p = 0.02).
Evidence suggests that patients with a preoperative breast cup size of C or larger may be at an increased risk for postoperative complication with immediate expander/implant BRs compared to those with a preoperative breast cup size of A or B [21]. A preoperative breast cup size larger than C remained a statistically significant risk factor for infection. Patients with a breast cup size of D or DD were nearly three times more likely than patients with smaller breasts to experience an infection (odds ratio 2.89 [95% CI 1.59–5.26]; p < 0.001). A retrospective comparative study observed a greater rate of skin necrosis in breasts larger than 600 g (> C cup) compared with breasts smaller than 600 g (A or B cup) (19% vs. 1.8%, respectively, p < 0.001) [22]. This may relate to tension in the wound close and on the flaps of heavier implants to some degree.
According to evidence, among patients with expander/implant BRs, diabetes is not a significant risk factor for postoperative complications, including implant failure, pulmonary embolism, seroma, necrosis, mastectomy flap necrosis, wound dehiscence, infection and capsular contracture or reconstructive failure, defined as the premature removal of expander or implant [21].
The review of Fisher and colleagues aimed to characterize factors associated with postoperative complications following breast reconstruction using the National Surgical Quality Improvement Program (ACS-NSQIP) database from 2005–2010 [22]. The database included either implant-based reconstruction (immediate, delayed and tissue expander) or autologous reconstruction (pedicled transverse rectus abdominus myocutaneous (TRAM) , free TRAM and latissimus dorsi flap with or without implant). During the study period, 16,063 breast reconstructions were performed. Autologous reconstructions were performed in 20.7% of patients and implant based in 79.3%. The incidence of major surgical complications was 8.4%, and the incidence of medical and wound complications was 1.6% and 3.5%, respectively. Independent risk factors for major surgical complications included immediate and autologous reconstructions, obesity, smoking, previous percutaneous cardiac surgery, recent weight loss, bleeding disorder, recent surgery, ASA ≥3, intraoperative transfusion and prolonged operative times. Risk factors for medical complications included autologous reconstruction, obesity, tumour involving CNS, bleeding disorders, recent surgery, ASA ≥3, intraoperative transfusion and prolonged operative times. Key identifiable risk factors associated with both surgical and medical morbidity included autologous breast reconstruction, obesity, ASA ≥3, bleeding disorders and prolonged operative time (◘ Table 28.3).
Table 28.3
Patient-related factors associated with major postoperative surgical complications in breast reconstruction according to the reviewed database (16,063 cases) of the National Surgical Quality Improvement Program (ACSNSQIP)
No complication | Major surgical complication | P-value | |||
|---|---|---|---|---|---|
N = 14,716 | N = 1347 | ||||
Number | Percent | Number | Percent | ||
Race | |||||
White | 11,587 | 78.7% | 1079 | 80.1% | 0.046 |
Black | 1007 | 6.8% | 110 | 8.2% | |
Asian | 402 | 2.7% | 22 | 1.6% | |
Hispanic | 139 | 0.9% | 19 | 1.4% | |
American Indian | 26 | 0.2% | 2 | 0.1% | |
Hawaii Indian | 30 | 0.2% | 2 | 0.1% | |
Unknown | 1525 | 10.4% | 113 | 8.4% | |
Age | n/a | 51.2 (10.6) | n/a | 51.6 (10.1) | 0.11 |
Immediate reconstruction | 12,451 | 84.6% | 1189 | 88.3% | <0.001 |
Type of reconstruction | |||||
Implant based | 11,767 | 80.0% | 962 | 71.4% | <0.001 |
Autologous | 2949 | 20.0% | 385 | 28.6% | |
Body mass index (kg/m2) (SD) | n/a | 27.0 (6.2) | n/a | 29.0 (7.1) | <0.001 |
Obesity (BMI > 30 kg/m2) | 3802 | 25.8% | 519 | 38.5% | <0.001 |
Diabetes | 692 | 4.7% | 81 | 6.0% | 0.03 |
Smoking | 1901 | 12.9% | 235 | 17.4% | <0.001 |
Alcohol use | 177 | 1.2% | 24 | 1.8% | 0.067 |
Dyspnoea | |||||
None | 14,174 | 96.3% | 1282 | 95.2% | 0.044 |
Moderate | 530 | 3.6% | 65 | 4.8% | |
At rest | 12 | 0.1% | 0 | 0.0% | |
Functional status | |||||
Independent | 14,676 | 99.7% | 1341 | 99.6% | 0.25 |
Partial dependence | 40 | 0.3% | 6 | 0.4% | |
History of chronic obstructive pulmonary disease | 126 | 0.9% | 12 | 0.9% | 0.9 |
History of congestive heart failure | 7 | 0.0% | 0 | 0.0% | 0.42 |
History of myocardial infarction | 2 | 0.0% | 0 | 0.0% | 0.67 |
Previous coronary intervention | 97 | 0.7% | 14 | 1.0% | 0.11 |
Prior cardiac surgery | 75 | 0.5% | 14 | 1.0% | 0.01 |
History of angina | 11 | 0.1% | 2 | 0.1% | 0.36 |
History of peripheral vascular disease | 14 | 0.1% | 4 | 0.3% | 0.034 |
Renal failure | 1 | 0.0% | 1 | 0.1% | 0.034 |
Dialysis | 10 | 0.1% | 1 | 0.1% | 0.93 |
Prior transient ischemic attack | 77 | 0.5% | 11 | 0.8% | 0.16 |
CNS tumour | 7 | 0.0% | 1 | 0.1% | 0.68 |
Active wound infection | 88 | 0.6% | 14 | 1.0% | 0.051 |
Current use of steroids | 132 | 0.9% | 15 | 1.1% | 0.42 |
Recent weight loss (>10% in 6 months) | 47 | 0.3% | 10 | 0.7% | 0.012 |
Bleeding disorder | 89 | 0.6% | 15 | 1.1% | 0.026 |
Chemotherapy in last 30 days | 639 | 4.3% | 46 | 3.4% | 0.11 |
Radiotherapy in last 30 days | 65 | 0.4% | 7 | 0.5% | 0.68 |
Operation within 30 days | 384 | 2.6% | 50 | 3.7% | 0.02 |
Malnutrition (albumin <3.5 g/dl) | 177 | 1.2% | 21 | 1.6% | 0.3 |
Haemoglobin (g/dl) | n/a | 13.3 (1.2) | n/a | 13.3 (1.3) | 0.3 |
Anaemia (haemoglobin <12 g/dl) | 1458 | 9.9% | 147 | 10.9% | 0.3 |
Major surgical complications were defined as a deep wound infection, graft or prosthetic loss or an unplanned return to the operating room within 30 days (◘ Table 28.4).
Risk factor | Measures of prevention |
|---|---|
Patient-related risk factors | |
Age >50 years | Autologous reconstruction to be considered |
Smoking | Advise against smoking for at least 2 weeks prior to and 2 weeks following surgery |
Hypertension | Ensure adequate medical treatment and optimize blood pressure |
Diabetes mellitus | Optimize blood glucose level (recommended interval: 4.4–6.1 mmol/L (79.2–110 mg/dL)) |
Obesity | Autologous reconstruction to be considered |
Hypercholesterolaemia | Encourage dietary changes and optimize cholesterol levels with medication if necessary |
Low white blood cell count | Achieve normal white blood cell count or consider autologous reconstruction |
Larger breast size | Autologous reconstruction to be considered |
Disease-related risk factors | |
Axillary lymph node dissection | Procedure to be performed in a separate session |
Mastectomy skin necrosis | Wound therapy. The implant should be placed submuscularly |
Immediate reconstruction | Delayed and/or autologous reconstruction to be considered |
Bilateral surgeries | Delayed and/or autologous reconstruction to be considered |
Therapy-related risk factors | |
Radiotherapy | Autologous reconstruction to be considered |
Chemotherapy | Intensive follow-up to detect infection in time |
Prolonged drain use | Early drain removal may help avoid infections |
Late expansion | Early tissue expansion is associated with early drain removal |
In case of failure of a previous reconstruction (complete failure or need for later substantial revision), DBR, using autologous flaps or reimplantation, may be necessary [24]. Indications for DBR include symptomatic capsular contracture, asymmetry, implant extrusion and exposure and previous partial or total flap loss.
28.3 Practical Considerations in Delayed Breast Reconstruction
28.3.1 Technical Assessment
Undoubtedly one of the skills of the oncoplastic surgeon is their ability to judge what will give a good aesthetic outcome for a particular woman, best fulfilling her wishes for the shape and volume of the new breast and how this relates to her body size and shape before surgery. For some women her contralateral current breast shape and size may not be her ideal, and many women wish for augmentation, reduction or correction of ptosis.
The assessment of other objective factors forms the next step in the assessment process: the records from previous surgery, length and position of scars, estimation of the weight and volume of resected tissue and skin [14]. The lack of an adequate skin envelope is a key consideration in DBR (◘ Fig. 28.5), and, in general, more skin and tissue volume are required from the flap that will be used for the reconstruction compared to IBR [11]. Finding enough skin to perform an adequate BR is usually not problematic if the patient is suitable for an abdominal pedicled or free flap (transverse rectus abdominis myocutaneous (TRAM) or DIEP flap) reconstruction, but it may pose an obstacle if the patient has a low BMI [11]. The surgeon needs to consider this preoperatively and plan the BR so that sufficient skin will be available. The surgeon should assess the texture and elasticity of the skin especially following RT [14]. Sun-damaged skin, chronic steroid consumption, heavy smoking or tattoos on the potential donor areas should be taken into consideration [11].
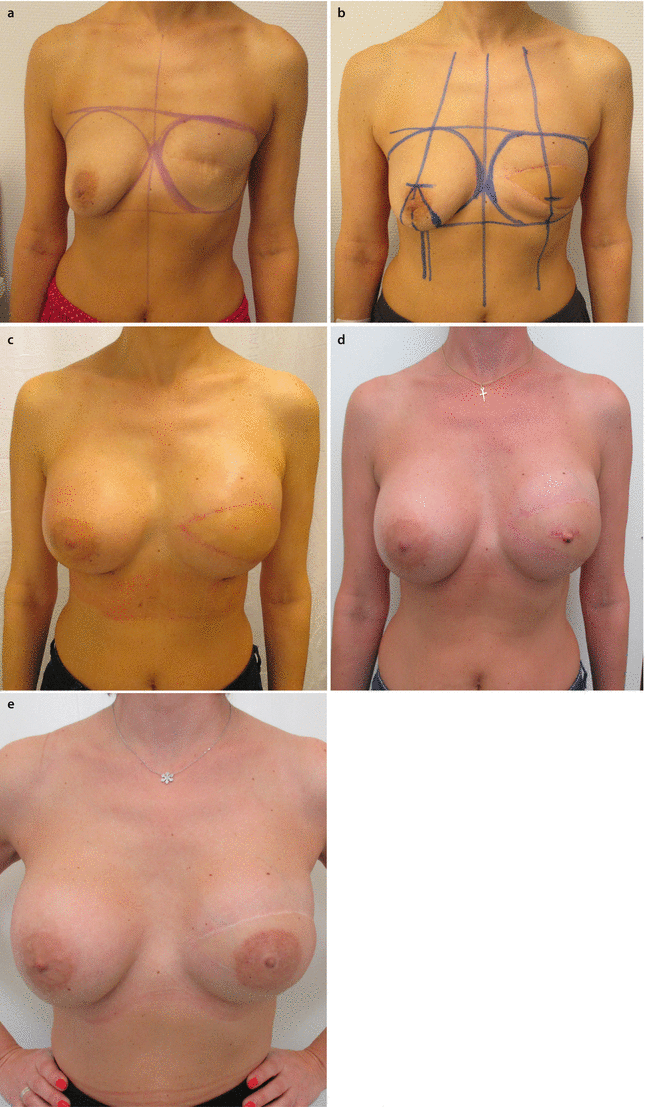

Fig. 28.5
a 41-year-old patent had a mastectomy and SLNB in 2013. b In 2014 an LDm c was performed on the left side. c Six months later the reconstruction was completed with the use of a textured, round ultrahigh profile 430 cm3 silicone implant, and for symmetrization a textured, round, high profile 300 cm3 was placed submuscularly on the right side. d, e The patient refused a mastopexy because of concerns about additional scars
The presence of scar tissue makes DBR complicated [11]. Scar tissue must be completely released so that the mastectomy flaps can expand to their original dimensions, only then may the missing tissue be accurately and successfully replaced [11].
The previously irradiated chest wall poses special surgical problems since chronic radiation damage leads to progressive fibrosis [9, 11]. Damaged, fibrotic tissues surrounding an autologous flap are less likely to blend into the tissues of the BR as well as they would without RT [11]. Radiation-damaged skin often needs to be discarded, and thus more skin may be required from the flap [11]. The quality of the aesthetic result that may be obtained in a patient who has had previous RT is therefore lower than that in a nonirradiated patient. If adequate information is provided such that patients have realistic expectations of the cosmetic end results, then disappointment may be avoided [11].
The estimation or objective measurement of breast volume using MR volumetry is very helpful in planning reconstruction, as well as the classification of the degree of breast ptosis. Ideally the volume of the resected breast should have been recorded at the time of mastectomy which is very helpful. There are numerous technologies available to permit calculation of breast volume, some based on MRI, 3D photography or on measured breast parameters. Simple in-clinic methods, such as use of a series of sizers or surgeon judgement, may be helpful but are less accurate and dependent on experience. The excised volume after BCS should be known from the pathological report but may be an underestimate as baseline breast size may have differed and subsequent radiotherapy may have caused further volume loss. The mean volume and size of the skin surface of the different autologous flaps have to be known for adequate planning of an autologous BR [11, 15, 25, 26] (◘ Table 28.5).
Name of the flap | Blood supply | Type of flap | Maximum size of skin island length × width (cm) | Surface of skin island (cm2) |
|---|---|---|---|---|
Thoracal flaps | ||||
Latissimus dorsi myocutaneous flap (LDmc) | Thoracodorsal artery | Pedicled or free | 35 × 12 | 420 |
Lateral thoracic flaps | Lateral thoracic or superficial thoracic artery | Pedicled | 15 × 11 | 165 |
Thoraco-epigastric flap | Lateral branch of superior epigastric artery, intercostal perforator artery | Pedicled | 15–22 × 8–12 | 120–264 |
Thoracodorsal artery perforator (TAP) flap | Thoracodorsal perforator artery and vein | Pedicled | 16–22 × 7–11 | 112–242 |
Intercostal artery perforator (ICAP) flap | Intercostal perforator artery | Pedicled | 22–26 × 6–8
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |




