Peritoneal metastases occur by “seeding” or direct contact unlike the more common hematogenous or lymphatic routes seen in most malignancies. This method of spread leads to considerable challenges in the management of patients due to difficulty in detection and quantification (staging) of burden of disease as there may be no identifiable mass formation on imaging studies. In addition, they can pose technical difficulties in the surgical extirpation of disease given that abdominal viscera are covered by a layer of visceral peritoneum which can be studded with disease. This leads to considerable discomfort and impairment of quality of life of patients via pain, ascites, cachexia, and malignant bowel obstruction, which ultimately leads to inanition and death. The application of cytoreductive surgery (CRS) and intraperitoneal therapies to this disease process offers an attractive method of tumor reduction and drug delivery that could overcome the drawbacks of delivery of intravenous therapy including tumor hypoxia and ischemia, and drug resistance leading to attrition of systemic dosing.1
Peritoneal disease comprises a heterogeneous group of malignancies with a common phenotypic expression of metastatic sites. Predominantly, there are two types of peritoneal disease: primary and secondary. Diseases arising from the peritoneum such as mesothelioma, desmoplastic round cell tumors, and primary peritoneal disease are rare. The majority of the tumors that present to the surgical oncologist are composed of secondary tumors, with an estimated incidence of 8% to 10% of all colorectal carcinomas, 30% of gastric carcinomas, and 60% to 70% of all ovarian carcinomas developing peritoneal disease (Table 122-1).2–4
Commonly Treated Histologies with Peritoneal Surface Disease
|
The gold standard for detection of peritoneal disease is pathological examination after visual suspicion during laparoscopy or laparotomy. Conventional imaging techniques for detection of peritoneal disease have poor discrimination of disease from normal peritoneum. This is due to either small size of tumor deposits or a sheet-like disease, which is difficult to detect on conventional optical interpretation of images. Improvements in imaging techniques including multi-slice CT scan and MRI have led to increased sensitivity of detection of peritoneal disease. The application of diffusion weighting to regular MRI techniques has been suggested to increase the sensitivity to 95% in patients with a high probability of disease.5 The application of PET imaging for detection of peritoneal disease is controversial. While the PET scan is useful in detecting metastatic disease in high-grade histologies, it is usually less sensitive in mucinous histologies, which are more likely to have peritoneal spread. In addition, sheets of tumor can often be mistaken for physiologic activity of the bowel or normal uptake by inexperienced teams. However, a negative PET scan is more likely to indicate a complete cytoreduction is possible.6,7
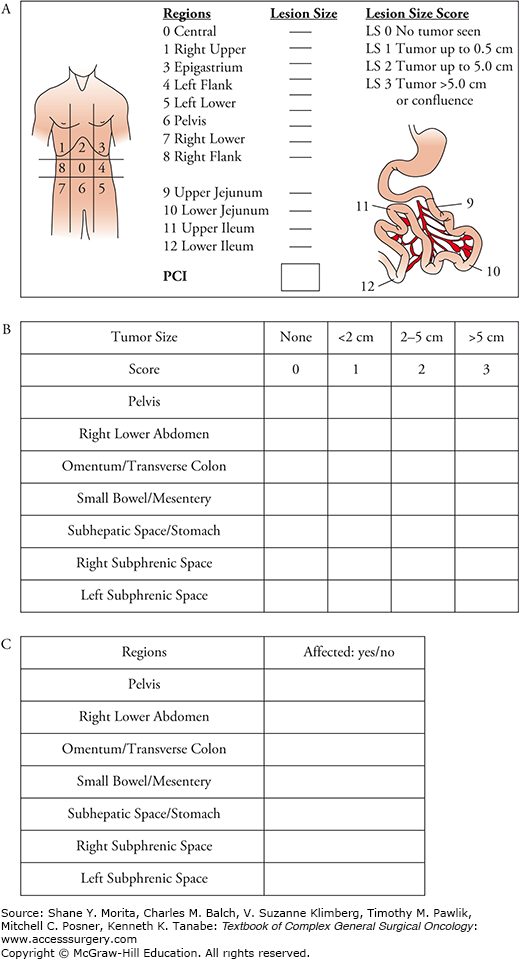
Upon detecting disease, there are numerous staging systems to determine the burden of disease. Staging systems that have been used include the peritoneal carcinomatosis index (PCI), simplified PCI, Gilly peritoneal score, Japanese gastric cancer P-Score, and the 7-region count.8–10 The most widely used staging system currently is the PCI system, which classifies tumors based on their size (score 0 to 3) and distribution in the peritoneal cavity which is divided in 13 zones (Fig. 122-1). The simplified PCI system is an easier to use scoring system that was found to be noninferior to the PCI score in prognostication.10 Both these scoring systems utilize a laparoscopy or a laparotomy to quantify the burden of disease. Close collaboration with a radiologist can help peritoneal malignancy programs generate an imaging PCI score. Scoring systems such as the peritoneal surface disease severity score (PSDSS) rely on distinguishing patients with high burden of disease on imaging. This score also includes histology and clinical symptoms to prognosticate and validation of the score is currently underway.11,12
FIGURE 122-1:
Scoring systems for peritoneal staging. A. PCI score. (Reproduced with permission from Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol. February 8, 2005;2(1):3.) B. Simplified PCI score. C. 7-Region count. (Reproduced with permission from Swellengrebel HA, Zoetmulder FA, Smeenk RM, et al. Quantitative intra-operative assessment of peritoneal carcinomatosis – a comparison of three prognostic tools. Eur J Surg Oncol. October 2009;35(10):1078–1084.)
Surgical management of patients with peritoneal disease follows either a curative or palliative paradigm. While all surgery for metastatic disease is sometimes considered palliative by some surgeons, long-term (>10 year) survival following CRS techniques has been shown in patients with appendiceal and colorectal cancers.13,14 This behooves the discerning surgical oncologist to define the goals of care and balance risks and benefits prior to therapy.
Not all patients are candidates for CRS, and several factors must be considered to decide whether cytoreductive techniques are appropriate. Some of the factors are elucidated in (Table 122-2).
Selection Criteria For Favorable Patients Undergoing Cytoreductive Surgery
| Selection Criteria for Favorable Patients Undergoing Cytoreductive Surgery |
| Host Factors |
| Good performance status (ECOG PS 0-2) |
| Low anesthetic risk (ASA 3 or less) |
| No significant organ failure (cardiac, pulmonary, hepatic, renal, and endocrine) |
| Adequate psychosocial coping mechanisms |
| Disease Factors |
| No extraperitoneal disease (limited disease in some cases) |
| No (or Limited) intrahepatic and para-aortic/paracaval nodal metastases |
| Favorable disease biology (e.g., responsiveness to systemic chemotherapy for high-grade histologies) |
| Low burden of disease (low PCI, PSDSS, etc.) |
| Technical Factors |
| Limited seromesenteric disease |
| Adequate functional length of bowel after cytoreduction (100-cm small bowel) |
|
| Low likelihood of postoperative complications |
| Primary surgery |
Patients are extensively counseled prior to undergoing CRS and intraperitoneal chemotherapy. It is important to emphasize goals of care and to provide information to the patients without offering unrealistic expectations of their care and recovery. Families of patients are encouraged to participate in the decision making if the patients desires it. Counseling regarding the need for stomas, sexual therapy, fertility preservation, and genetic counseling as appropriate is especially important in the young patients. Referral to patient support groups can be helpful. Alterations in daily living, advanced care planning, and need for assisted living care are better focused in the geriatric population. The risk of mortality is low from modern CRS techniques, and the authors recommend an elaborate discussion on the morbidity of the surgery. Recent imaging, colonoscopy and endoscopy, and tumor markers as appropriate are critical before the operation. Discussion regarding preoperative anesthesia, supportive oncology (or palliative care), and advanced care planning can be extremely helpful. The role of enhanced recovery after surgery (ERAS) pathways and “prehabilitation” programs are currently being investigated.
Most patients need to be in peak physical condition in order to proceed with surgery. Physical activity and nutrition are optimized prior to the operating room. While mechanical bowel preparation can be helpful for maintaining a clean field, some authors have suggested the occurrence of significant fluid shifts during the operation.15 Studies from colorectal literature suggest no benefit and perhaps even a deleterious effect of the bowel preparation.16 There is no consensus regarding routine use of mechanical bowel preparation in patients undergoing CRS.
Patients are adequately padded during the operation. Due to the long nature of the operation, patients are kept either supine or in split leg position when access to the perineum is needed. Some institutions keep patients in low lithotomy during the entire cytoreduction after ensuring adequate padding of nerves and frequent checking for compartment syndrome in the stirrups. Patients can be offered epidural catheters, which provide excellent regional analgesia after surgery. Some authors have suggested that early continuous infusion of the epidural during surgery might reduce blood loss.17 Concerns for loss of sympathetic tone must be carefully assessed when severe hypotension occurs during the operation. Anesthetic and fluid management is critical during the operation, and communication is essential. Measures are taken to prevent deep vein thrombosis (sequential compressive devices and preoperative heparin) and maintain normothermia. Antibiotics administered must be monitored to avoid overdosing (when redosed frequently) or underdosing (when encountering massive blood loss). Colloid replacement can be used for high-volume ascites during the operation.
The goal of CRS is to remove all visible disease or cytoreduce it to less than 2.5 mm. Although some histologies (such as ovarian) have accepted residual disease burden of 1 cm or less as optimal, numerous reports validate better outcomes for completely cytoreduced patients. Certain unique oncologic aspects of surgery are described below.
Laparoscopy is generously utilized in the management of patients undergoing CRS.18,19 While the sensitivity of laparoscopy compared to a systematic laparotomy has never been compared, it is inherent in the technique that unless performed diligently one is likely to understage patients. However, it is a valuable exclusion tool, especially for patients who may benefit from chemotherapy first approaches. It is usually performed prior to the operation or as a separate staging procedure. The open hasson and the optical trocar technique are the most frequent methods of entry into the peritoneal cavity.
Unlike other oncologic surgery, in patients with low-grade histology with visceral peritoneal involvement, decision making regarding visceral preservation is critical to the conduct of the operation. Resection of serosal nodules, and stripping of glissons capsule, bladder, ureters, uterus, and other viscera are particularly helpful techniques. In high-grade histologies with low burden of disease, complete visceral resection could offer patients a better chance of survival.
Techniques regarding peritonectomy and omentectomy have been described elsewhere by us and others.19,20 Complete resection of diseased peritoneum while maintaining oncological principles offers patients the best chance of a complete cytoreduction. Complete peritonectomy might not be necessary when only limited disease involvement is noted. Routine resection of the falciform ligament and ligamentum teres is recommended, and the pont hepatique is opened.21 The details of the surgical techniques and their pitfalls are described in Table 122-3.
Techniques of Peritonectomy and Potential Pitfalls
| Technique | Details | Pitfalls |
|---|---|---|
| Peritonectomy | ||
| Right diaphragmatic |
|
|
| Left diaphragmatic |
|
|
| Right and left paracolic gutter |
|
|
| Pelvic peritonectomy |
|
|
| Anterior peritonectomy |
|
|
| Omentectomy—greater |
|
|
| Omentectomy—lesser |
|
|
| Superior omental recess/IVC bursa |
|
|
| Peri-portal omentum/vestibule |
|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






