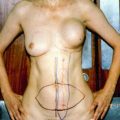
Fig. 6.1
Algorithm for the management of nipple discharge. *If patient plans future breastfeeding, selective duct excision is preferred over major duct excision
The characteristics of the discharge should be obtained and recorded in detail with an attempt to categorize whether it is due to lactation, galactorrhea, or pathologic discharge. The clinician should be sure to understand if the discharge is spontaneous or induced, unilateral or bilateral, the characteristics of the discharged fluid (including volume), the frequency of the discharge, and whether the patient is stimulating his or her nipple to examine for discharge. This latter factor is important as regular self-examination for discharge can produce ongoing, even spontaneous, discharge. Regular self-examination or other forms of breast stimulation can repress the secretion of hypothalamic prolactin inhibitory factor, resulting in hyperprolactinemia and galactorrhea [16].
The physical examination should include careful inspection of the breast skin, nipple, and areola as well as palpation of all the breast parenchyma, including the subareolar tissue and the regional lymph nodes. Care should be taken to examine the nipple for evidence of a central horizontal crease that is associated with duct ectasia, an entity which can also produce nipple discharge. Careful pressure can be exerted at the areolar margin circumferentially to examine for discharge. The discharged fluid can then be inspected for origin from a single or multiple ducts, color, and texture (thin, thick, sticky, etc.).
Hemoccult testing of the discharge is not usually performed, as both serous and bloody discharge can be associated with an underlying breast carcinoma [9, 20]. Cytologic analysis is not regularly performed, as the results of such studies are neither sensitive nor specific for an underlying breast cancer [9, 20–23]. Among patients with biopsy proven carcinoma, 29 % of cytology specimens of the discharge have been reported to show no evidence of carcinoma or atypia [24]. If the patient is found to have subareolar tenderness and periareolar erythema with purulent nipple discharge, this is consistent with a subareolar abscess rather than true nipple discharge. These patients are obviously approached differently and should be treated with an appropriate combination of antibiotics and possible incision and drainage and/or an excision of the subareolar major ducts [25].
Imaging and Laboratory Investigations in Nipple Discharge
There are no radiologic studies that are essential, except for routine screening mammography, when the history and physical examination reveals that the discharge has characteristically benign features (Fig. 6.1). Patients with lactation discharge need no further evaluation, including those with occult or gross blood in the discharged milk. Patients with galactorrhea need no further evaluation for breast carcinoma, but should be evaluated for an underlying cause of hyperprolactinemia including a careful review of medications, review of the patient’s history for possible causes of neurogenic stimulation of the nipple-areola complex that would represses the secretion of hypothalamic prolactin inhibitory factor, and review of the history and physical examination for signs or symptoms of pituitary adenoma [16]. One may then perform laboratory workup of the galactorrhea with serum prolactin levels, though the serum prolactin concentration is normal in nearly half of women who present with galactorrhea [26]. Galactorrhea in the absence of hyperprolactinemia is usually not the result of any ongoing disease process.
For those patients with pathologic nipple discharge and without clearly benign features (Fig. 6.1), we proceed to diagnostic mammography (for those 30 years of age and older) and subareolar ultrasound. These imaging modalities have been reported to be able to separate patients with a high risk of underlying carcinoma from those with a low risk (Table 6.1) [17, 6, 8, 9, 11, 20, 27]. The risk of carcinoma with pathologic nipple discharge and an abnormal mammogram, while an uncommon scenario, is as high as 60 %, and the risk with an abnormal ultrasound but normal mammogram is 7 % (Fig. 6.2, Table 6.2) [17].
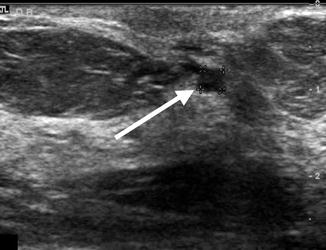
Table 6.1
Comparative rates of carcinoma risk
Characteristic | Carcinoma rates (%) | p |
|---|---|---|
Age ≥50 vs. <50 years | 6 % vs. 0 % | 0.02 |
Unilateral vs. bilateral discharge | 4 % vs. 2 % | 0.49 |
Spontaneous vs. non-spontaneous | 5 % vs. 0 % | 0.13 |
Serous/bloody vs. other discharge | 5 % vs. 0 % | 0.10 |
Abnormal vs. normal mammogram | 38 % vs. 3 % | <0.01 |
Abnormal vs. normal ultrasound | 12 % vs. 1 % | <0.01 |
Abnormal vs. normal ductogram | 6 % vs. 0 % | 0.64 |

Fig. 6.2
Subareolar ultrasound demonstrating a 0.35 cm intraductal lesion (arrow) in a patient subsequently found to have ductal carcinoma in situ upon subareolar duct excision
Table 6.2
Cancer risk by clinical scenario
Clinical scenarioa – nipple discharge with | N | Risk of carcinoma (%) | Risk in other reports |
|---|---|---|---|
All patients with nipple discharge | 204 | 3 | |
Nipple discharge, underwent biopsy | 75 | 9 | |
Non-spontaneous discharge | 49 | 0 | |
Bilateral discharge | 52 | 2 | |
Unilateral, spontaneous, serous discharge from single duct | 49 | 4 | |
Unilateral, spontaneous, bloody discharge from single duct | 60 | 7 | |
Unilateral bloody/serous discharge, single duct, and negative mammogram | 106 | 3 | 3 % [11] |
Unilateral bloody/serous discharge, single duct, negative mammogram and negative ultrasoundb | 57 | 0 | 3 % [1] |
Unilateral bloody/serous discharge, single duct, negative mammogram and abnormal ultrasound | 30 | 7 | |
Unilateral bloody/serous discharge, single duct, and abnormal mammogram | 5 | 60 | 13 % [11] |
Ductography can be helpful in the evaluation of pathologic nipple discharge, though the use of subareolar ultrasound in skilled hands greatly minimizes the additional diagnostic yield of ductography. At our institution, we seldom use ductography as a diagnostic tool but rather to provide a “roadmap” as needed for subareolar duct excision once a decision has been made to perform this operation (Fig. 6.1). The primary benefit of ductography is to localize the lesion, especially in the case of multiple and peripheral lesions [4, 20, 28–32]. This allows radiologic guidance, such as wire or radioactive seed localization [33], to be used to direct the major duct excision and be certain that the area/lesion is completely resected.
Of note, ductography has been reported to miss as many as 20 % of ductal lesions, including those of a benign nature [4]. Although the negative predictive value is relatively high (82–91 %) [4, 9, 20, 31], it is still not sensitive enough to exclude the possibility of malignancy. In one series of 163 patients, ductography was associated with a sensitivity of 76 %, a specificity of 11 %, and a positive predictive value of only 19 % [34]. Such performance of this test makes it difficult to justify regularly subjecting patients to a sometimes painful procedure if reliable subareolar ultrasound is available.
The role of ductoscopy in nipple discharge remains to be defined. While this procedure holds some promise, the presence of cancer has been reported to predict unsuccessful ductal cannulation with the ductoscope [27]. Ductoscopy-guided excision, like ductograpy-guided excision, has been reported to increase the yield of atypia or carcinoma in at least one series [35]. Among 114 women in which half the patients were evaluated with ductoscopic guidance and half with surgery alone, the yield of pathologic diagnoses did not significantly differ between the groups [35]. In addition, ductoscopy was technically unsuccessful in 13 % of patients [35]. Currently, we believe that ductoscopy adds little diagnostic value in nipple discharge, with further refinements in instrumentation and technique possibly increasing its usefulness in the future.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree