Serotype
Species and varieties
Molecular types
A
C. neoformans var. grubii
VN I, VN II, VN B
B
C. gattii
VG I, VG II, VG III, VG IV
C
C. gattii
VG I, VG II, VG III, VG IV
D
C. neoformans var. neoformans
VN IV
AD
C. neoformans
VN III
The life cycle of C. neoformans and C. gattii involve asexual (yeast) and sexual (basidiospores/hyphae) forms. The asexual form is the encapsulated yeast that reproduces by narrow-based budding and is found most commonly in clinical specimens, whereas the sexual stage, which exists in one of two mating types, “alpha” or “a,” is observed only under certain conditions, resulting in meiosis to form basidiospores. The vast majority of clinical infections and environmental isolates are caused by “alpha” mating-type locus strains. Since the sexual stage of C. neoformans and C. gattii has been described, their teleomorphs were named Filobasidiella neoformans and Filobasidiella bacillospora, respectively.
C. neoformans and C. gattii usually appear as white-to-cream, opaque, and mucoid colonies that grow to several millimeters in diameter on the most routine agar within 48–72 h. With some strains, a few colonies occasionally develop sectors with different pigmentation or different morphologies (i.e., wrinkled, smooth, mucoid). Both cryptococcal species will grow readily on most fungal culture media without cycloheximide at 30–37 °C in aerobic conditions. However, C. neoformans is generally more thermotolerant than C. gattii, and, within this species, serotype A is generally more tolerant than serotype D strains. In addition to their ability to grow at 37 °C, the yeast produce a thick shedding polysaccharide capsule, melanin pigments, and the enzymes urease and phospholipase, which allow Cryptococcus to be readily identified from other yeasts. These are also considered to be yeast virulence factors.
Epidemiology
Cryptococcosis was considered an uncommon infection prior to the AIDS epidemic, associated with malignancies, organ transplantation, and certain immunosuppressive treatments. Beginning in the early 1980s, the incidence of cryptococcosis increased significantly and between 6 and 10 % of persons with AIDS developed cryptococcosis [7, 8]. In fact, HIV/AIDS was found to be associated with 80 % of cryptococcosis cases worldwide. Cryptococcal infection is a major OI in HIV-infected patients as the CD4+ cell count falls below 100 cells/µl. Following widespread implementation of HAART, the incidence of cryptococcosis among patients with HIV/AIDS has fallen significantly in most developed nations. The incidence of cryptococcal infection in persons not infected with HIV has remained stable during this time. Moreover, in developing nations with limited access to HAART, the prevalence of and morbidity and mortality associated with cryptococcosis remains unacceptably high, accounting for up to 600,000 deaths per year. Besides HIV infection, other risk factors for acquiring cryptococcal infections include many conditions that result in an immunocompromised status (Table 15.2). Although both C. neoformans and C. gattii can cause cryptococcosis in apparently normal hosts, the percentage of C. gattii infections causing disease in such patients is significantly higher than for C. neoformans.
Table 15.2
Predisposing factors of cryptococcosis
HIV infection Malignanciesa (e.g., Hodgkin’s disease, other lymphomas, and chronic lymphocytic leukemia) Lymphoproliferative disordersa Idiopathic CD4+ T cell lymphopenia Rheumatologic or immunologic diseasesa Sarcoidosis Systemic lupus erythematosus Rheumatoid arthritis Hyper-IgM syndrome or hyper-IgE syndrome Monoclonal antibodies (etanercept, infliximab, alemtuzumab) Corticosteroid and/or immunosuppressive therapies Diabetes mellitus Solid organ transplantationa Chronic pulmonary diseases Renal failure and/or peritoneal dialysis Chronic liver diseasesb |
C. neoformans is found throughout the world in association with excreta from certain birds such as pigeons and in tree hollows. C. gattii is commonly associated with several species of eucalyptus and other trees [9]. While the link between the environmental source of infection and cryptococcosis cases is not precise, there is evidence to suggest an increased risk of cryptococcosis and asymptomatic cryptococcal antigenemia following intense bird exposures. Recently, there has been a strong link between the C. gattii outbreak in humans on Vancouver Island and common environmental yeast exposures. Although these fungi can be detected in endobronchial specimens from humans without disease (colonization), clinicians should be alert for subclinical disease or potential for disease when Cryptococcus is isolated from any clinical specimen.
Approximately 95 % of cryptococcal infections are caused by serotype A strains (C. neoformans var. grubii) with the remaining 4–5 % of infections caused by serotype D (C. neoformans var. neoformans) or serotype B and C strains (C. gattii). Whereas C. neoformans serotype A is found worldwide, serotypes B and C are found primarily in tropical and subtropical regions such as southern California, Hawaii, Brazil, Australia, Southeast Asia, and central Africa (and more recently identified in temperate climates such as Vancouver Island and the Pacific Northwest region of the USA), and serotype D is predominantly found in European countries (Table 15.3) [10]. In Australia and New Zealand, serotypes B and C caused up to 15 % of all cases of cryptococcosis cases in one study, but serotype A remains the predominant serotype even in these endemic areas [11]. To date, only C. gattii strains have been reported to cause a widespread defined outbreak of disease [5].
Table 15.3
Distribution of C. neoformans and C. gatti
Cryptococcus species | Primary areas of distribution |
|---|---|
C. neoformans var. grubii serotype A | Worldwide; pigeon guano, tree hollows |
C. gattii | Tropical and subtropical regions: southern California, Hawaii, Brazil, Australia, Southeast Asia, and central Africa; eucalyptus trees, firs, and oak trees |
C. neoformans var. neoformans serotype D | Europe: Denmark, Germany, Italy, France, and Switzerland; less common in the environment than serotype A |
Pathogenesis and Immunology
Cryptococcosis occurs primarily by inhalation of the infectious propagules, either dehydrated (poorly encapsulated) yeasts or basidiospores, into pulmonary alveoli. Direct inoculation into tissue due to trauma can be a portal of entry in occasional cases and, potentially, yeast may enter through the gastrointestinal tract. After the yeasts are inhaled into the lungs of a susceptible host, they encounter alveolar macrophages, and other inflammatory cells are recruited through release of cytokines and chemokines such as interleukin (IL)-12, IL-18, monocyte chemotactic protein (MCP)-1, and macrophage inflammatory protein (MIP)-1α. Cryptococcal infection primarily involves granulomatous inflammation, which is a result of a helper T cell (Th1) response with cytokines including tumor necrosis factor, interferon-γ, and IL-2 [12]. In many circumstances, the yeasts remain dormant (yet viable) in hilar lymph nodes or pulmonary foci of an asymptomatic individual for years and then disseminate outside those complexes when local immunity is suppressed, similar to that which is observed in cases of reactivation tuberculosis or histoplasmosis [10]. In a patient with severely compromised cellular immunity, the yeasts reactivate and proliferate at the site of infection and then disseminate to other sites causing progressive clinical symptoms.
Recent advances in the molecular biology of Cryptococcus have confirmed several virulence factors. The three classical virulence factors of C. neoformans include: capsule formation, melanin pigment production, and the ability to grow well at 37 °C [9, 12]. The prominent antiphagocytic polysaccharide capsule, which is composed of glucuronoxylomannan (GXM), is unique to Cryptococcus species and is considered an essential virulence factor that has multiple effects on host immunity. In addition, C. neoformans possesses an enzyme that catalyzes the conversion of diphenolic compounds to form melanin, which may have a biological role to protect the yeasts from host oxidative stresses and which may partially explain the organism’s neurotropism. Finally, its ability to grow at 37 °C is a basic part of the virulence composite for most of the human pathogenic fungi including Cryptococcus, as molecular studies have linked high-temperature growth with certain signaling pathways and enzymes that this yeast has acquired or adapted over time in order to enhance its pathogenicity. Other virulence factors include phospholipase and urease production and multiple enzymes associated with protection against oxidative stresses.
Clinical Manifestations
C. neoformans and C. gattii have a predilection for establishing clinical disease in the lungs and central nervous system (CNS). Other organs that may be involved in cryptococcosis include skin, prostate, eyes, bone, and blood [2, 8, 10, 13]. In fact, this yeast may cause disease in any organ of the human body, and widely disseminated cryptococcal infection can affect multiple organs in severely immunosuppressed patients (Table 15.4).
Table 15.4
Clinical manifestations of cryptococcosis. (Adapted from Casadevall, A, Perfect, JR. Cryptococcus neoformans. Washington: ASM Press; 1998: 409 [2])
Organs | Common clinical manifestations |
|---|---|
Central nervous system | Acute/subacute/chronic meningoencephalitis Cryptococcomas (abscesses) Spinal cord granuloma Chronic cognitive impairment (sequelae of hydrocephalus) |
Lung | Asymptomatic airway colonization Pulmonary nodule(s) Hilar or mediastinal lymphadenopathy Lobar/interstitial infiltrates Miliary infiltrates Lung cavities Endobronchial lesions Pleural effusion/empyema Pneumothorax Acute/subacute pneumonia Acute respiratory distress syndrome |
Skin | Papules with central ulceration (molluscum contagiosum-like) Subcutaneous abscesses Nodules/papules Cellulitis Draining sinuses Ulcers |
Eye | Papilledema Endophthalmitis Optic nerve atrophy Chorioretinitis Keratitis Paresis of extraocular muscles |
Genitourinary tract | Prostatitis Cryptococcuria Renal abscess Genital lesions |
Bone and joints | Osteolytic lesion(s) Arthritis (acute/chronic) |
Cardiovascular system | Cryptococcemia Endocarditis (native/prosthetic) Mycotic aneurysm Myocarditis Pericarditis |
Other organs | Myositis Peritonitis Hepatitis Nodular/ulcerative GI mucosal lesions Pancreatic mass Breast abscess Adrenal mass and adrenal insufficiency Thyroiditis or thyroid mass Sinusitis Salivary gland enlargement |
Pulmonary Infection
The respiratory tract serves as the most important portal of entry for this yeast, and thus there are many clinical manifestations of pulmonary cryptococcosis, ranging from asymptomatic transient or chronic colonization of the airways or simply a pulmonary nodule on radiograph to life-threatening fungal pneumonia with acute respiratory distress syndrome (ARDS) [2, 8]. In a normal host with cryptococcal infection, asymptomatic pulmonary cryptococcosis can occur in about one third of patients with pulmonary infection and patients may present to care with only an abnormal chest radiograph. The most common radiologic findings of cryptococcosis include well-defined single or multiple noncalcified nodules (Fig. 15.1) and pulmonary infiltrates (Fig. 15.2), but other less frequent radiographic findings include pleural effusions, hilar lymphadenopathy, and lung cavitation. Patients with pulmonary cryptococcosis can present with symptoms of acute onset of fever, productive cough, respiratory distress, chest pain, and weight loss [14]. The outbreak of C. gattii infections in Vancouver Island included several cases of severe symptomatic pulmonary cryptococcosis in apparently immunocompetent individuals. In an immunocompromised patient, especially those with HIV infection, cryptococcal pneumonia is usually symptomatic and can progress rapidly to ARDS, even in the absence of CNS involvement. Most immunocompromised patients with cryptococcal infection, however, present with CNS rather than pulmonary symptoms. In fact, more than 90 % of HIV/AIDS patients with cryptococcal infection already have CNS cryptococcosis at the time of diagnosis, many of whom will have a paucity of respiratory complaints. The findings in chest radiographs of immunocompromised patients with pulmonary cryptococcosis are the same as those in immunocompetent patients, but alveolar and interstitial infiltrates tend to be more frequent and imaging can mimic Pneumocystis pneumonia. Accelerated presentations of cryptococcal pneumonia are more common among immunocompromised patients. In pulmonary cryptococcosis, if the infection is confined to the lung, serum cryptococcal polysaccharide antigen is usually negative. However, while a positive serum polysaccharide antigen may indicate the dissemination of the yeast from the lung, it does not confirm CNS involvement. In immunocompromised individuals with pulmonary cryptococcosis, a lumbar puncture to rule out CNS disease should be considered regardless of the patient’s symptoms or serum polysaccharide antigen test results. The only setting in which screening a lumbar puncture may not necessarily need to be performed in a patient with Cryptococcus isolated from the lung is in the asymptomatic, immunocompetent patient with disease that appears to be limited to the lungs.
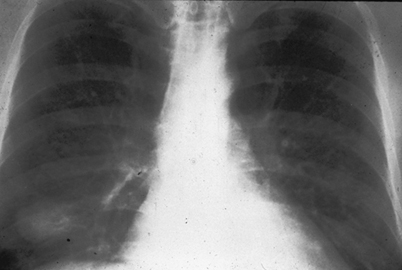
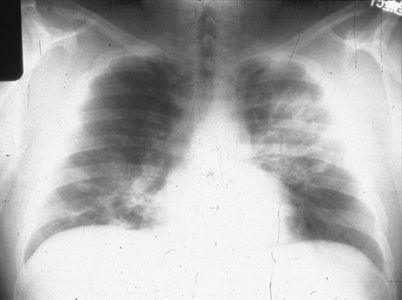

Fig. 15.1
Chest radiograph of pulmonary cryptococcosis presents as a single nodule in the lung at right lower lung field. (From A. Casadevall and J. R. Perfect, Cryptococcus neoformans, ASM Press, 1998. Reprinted with permission from Oxford University Press)

Fig. 15.2
Chest radiograph of pulmonary cryptococcosis presents as left lobar infiltrates. (From A. Casadevall and J. R. Perfect, Cryptococcus neoformans, ASM Press, 1998. Reprinted with permission from Oxford University Press)
CNS Infection
Clinical manifestations of CNS cryptococcosis include headache, fever, cranial neuropathy, alteration of consciousness, lethargy, memory loss, and signs of meningeal irritation [2, 8]. These findings are usually present for several weeks and therefore cause a clinical syndrome of subacute meningitis or meningoencephalitis. However, on some occasions, patients can present more acutely or lack typical features including headache. In HIV-infected patients with CNS cryptococcosis, the burden of fungal organisms in the CNS is usually high. Therefore, these patients may have a shorter onset of signs and symptoms, higher cerebrospinal fluid (CSF) polysaccharide antigen titers and intracranial pressures (ICPs), and slower CSF sterilization after starting antifungal treatment.
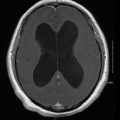
Different species may produce differences in clinical manifestations. For instance, one species may have a predilection to cause disease in brain parenchyma rather than the meninges. In certain areas of the world, C. gattii tends to cause cerebral cryptococcomas (Fig. 15.3) and/or hydrocephalus with or without large pulmonary mass lesions more frequently than C. neoformans. These patients with brain parenchymal involvement usually have high ICP and cranial neuropathies, and respond poorly to antifungal therapy.
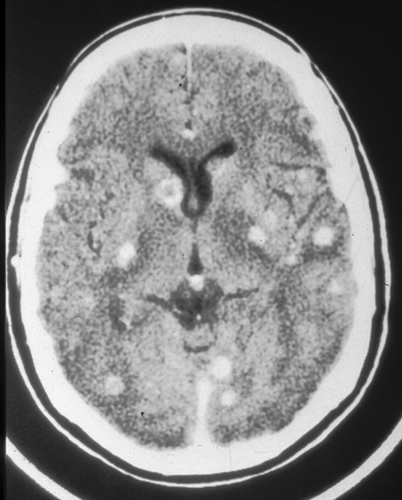

Fig. 15.3
CT scan of the brain showing multiple cryptococcomas in an apparently normal host. CT computed tomography. (From A. Casadevall and J. R. Perfect, Cryptococcus neoformans, ASM Press, 1998. Reprinted with permission from Oxford University Press)
Skin Infection
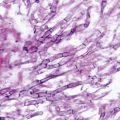
Cutaneous infections are the third most common clinical manifestations of cryptococcosis. Patients can manifest several types of skin lesions. One common skin lesion is a papule or maculopapular rash with central ulceration that may be described as “molluscum contagiosum-like.” These lesions are indistinguishable from those due to other fungal infections including Histoplasma capsulatum, Coccidioides immitis, and Penicillium marneffei. Other cutaneous lesions of cryptococcosis include acneiform lesions, purpura, vesicles, nodules, abscesses, ulcers (Fig. 15.4), granulomas, pustules, plaques, draining sinus, and cellulitis. Because there are many skin manifestations in cryptococcosis that mimic other infectious as well as malignant conditions, skin biopsy with culture and histopathology are essential for definitive diagnosis. Skin lesions of cryptococcosis usually represent disseminated cryptococcal infection. Primary cutaneous cryptococcosis is very rare and is usually associated with skin injury and direct inoculation of the yeasts. Solid organ transplant (SOT) recipients on tacrolimus seem to be more likely to develop skin, soft-tissue, and osteoarticular infections due to Cryptococcus [15]. Tacrolimus has anti-cryptococcal activity at high temperatures, but loses this activity as environmental temperatures decrease; this may in part explain the increased frequency of cutaneous cryptococcosis in these patients. Despite this series of patients, however, the most common site of disseminated infection in SOT recipients still remains the CNS, including patients receiving tacrolimus.
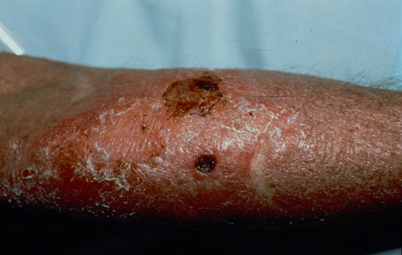

Fig. 15.4
Skin ulceration and cellulitis as cutaneous cryptococcosis
Prostate Infection
Prostatic cryptococcosis is usually asymptomatic, and the prostate gland is considered to be a sanctuary site for this yeast. The prostate may serve as an important reservoir for relapse of cryptococcosis in patients with a high fungal burden [16]. Latent C. neoformans infection has even been recognized to disseminate to the bloodstream during urological surgery on the prostate [17]. Cultures of urine or seminal fluid may still be positive for Cryptococcus after initial antifungal treatment of cryptococcal meningitis in AIDS patients [18], strongly supporting the need for prolonged antifungal treatment to clear the prostate in these severely immunocompromised patients.
Eye Infection
In the early reports of cryptococcal meningitis before the AIDS epidemic, ocular signs and symptoms were noted in approximately 45 % of cases [19]. The most common manifestations were ocular palsies and papilledema. In the present HIV era, several other manifestations of ocular cryptococcosis have been identified, including the presence of extensive retinal lesions with or without vitritis, which can lead to irreversible blindness. Furthermore, catastrophic loss of vision without evidence for endophthalmitis has also been reported [20]. Visual loss may be due to one of two pathogenic processes. The first is caused by infiltration of the optic nerve with the yeasts, producing rapid visual loss with few effective treatments. The second is due to increased ICP and compression of the ophthalmic artery. In this setting, patients have slower visual loss and treatment with serial lumbar punctures or ventricular shunts can prevent or slow down visual loss.
Infection at Other Body Sites
In addition to lung, CNS, skin, prostate, and eye, C. neoformans can cause disease in many other organs (Table 15.4). Cryptococcemia can occur in severely immunosuppressed patients but rarely causes endocarditis. Bone involvement of cryptococcosis typically presents as one or more circumscribed osteolytic lesions in any bone of the body, occasionally associated with “cold” soft-tissue abscesses, and has been associated with sarcoidosis. Bone marrow infiltration can be observed in severely immunocompromised hosts. Cryptococcal peritonitis [21] and cryptococcuria are also reported in several case series. Any organ of the human body can be a site of cryptococcal infections.
Diagnosis
There are several methods used for the diagnosis of cryptococcosis. These techniques include direct examination of the fungus in body fluids, histopathology of infected tissues, serological studies, and culture of body fluids or tissues. Molecular methods, while available, are not currently used in routine clinical practice.
Direct Examination
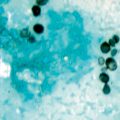
The most rapid method for diagnosis of cryptococcal meningitis is direct microscopic examination for encapsulated yeasts by an India ink preparation of CSF. Cryptococcus can be visualized as a globular, encapsulated yeast cell with or without budding, ranging in size from 5 to 20 µm in diameter. It is easily distinguished in a colloidal medium of India ink when mixed with CSF (Fig. 15.5). Approximately 1–5 mL of specimen is recommended for use in the India ink preparation. India ink examination can detect encapsulated yeasts in a CSF specimen with a threshold between 103 and 104 colony-forming units of yeasts/mL of fluid. The sensitivity of the India ink preparation technique is 30–50 % in non-AIDS-related cryptococcal meningitis and up to 80 % in AIDS-related disease. Some false-positive results can be found from intact lymphocytes, myelin globules, fat droplets, and other tissue cells. Also, dead yeast cells can remain in the CSF and be visualized by India ink preparation for varying periods of time during and after appropriate antifungal treatment. This is a limitation of direct microscopy of CSF during the management of cryptococcal meningitis [22].
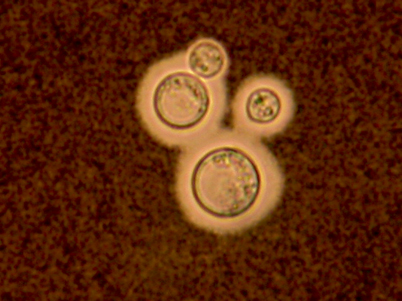

Fig. 15.5
India ink preparation showing budding encapsulated yeasts of Cryptococcus neoformans
Cytology and Histopathology
Cryptococcus can be identified by histological staining of tissues from lung, skin, bone marrow, brain, or other organs [23]. Histopathological staining of centrifuged CSF sediment is more sensitive for rapid diagnosis of cryptococcal meningitis than the India ink method [24]. Peritoneal fluid from chronic ambulatory peritoneal dialysis, seminal fluid, bronchial wash, or bronchoalveolar lavage fluid can also be used for cytology preparations in the diagnosis of cryptococcal infections, whereas India ink preparations from these body fluids are difficult to interpret [25, 26]. Fine needle aspiration for cytology of peripheral lymph nodes, adrenal glands, or vitreous aspiration; percutaneous transthoracic biopsy under real-time ultrasound guidance; or video-assisted thoracoscopic lung biopsy on pulmonary nodules, masses, or infiltrative lesions can be used for obtaining tissues for cytology/histopathology [27].
A variety of positive staining methods have been described to demonstrate the yeast cells in tissue or fluids, ranging from the nonspecific Papanicolaou or hematoxylin and eosin stains to the more specific fungal stains such as Calcofluor, which binds fungal chitin, or Gomori methenamine silver (GMS), which stains the fungal cell wall [2, 25] (Fig. 15.6). Several stains can identify the polysaccharide capsular material surrounding the yeasts. These stains can be especially useful in presumptively identifying Cryptococcus when the organism does not grow or cultures are not obtained. They include Mayer’s mucicarmine, periodic acid-Schiff (PAS), and alcian blue stains [2]. The Fontana-Masson stain appears to identify melanin in the yeast cell wall. The fungus is observed as a yeast that reproduces by formation of narrow-based budding with a prominent capsule. Gram stain is not optimal for identification of this yeast, but may show C. neoformans as a poorly stained gram-positive budding yeast (Fig. 15.7) [2]. The recognition of C. neoformans in gram-stained smears of purulent exudates may be hampered by the presence of the large gelatinous capsule that apparently prevents definitive staining of the yeast-like cells.
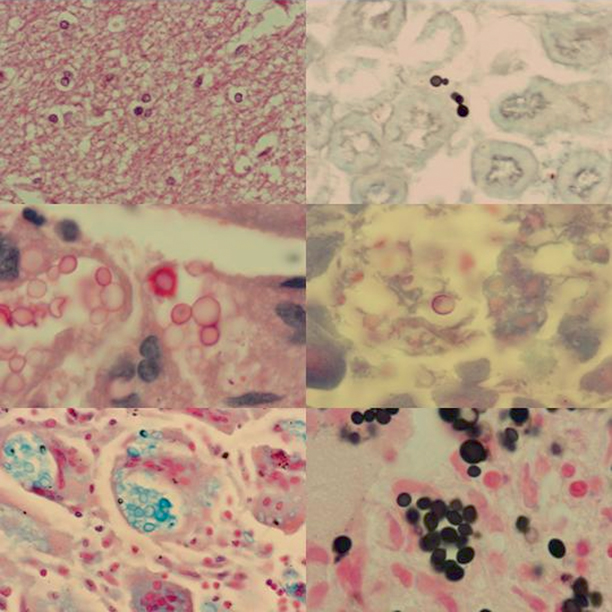
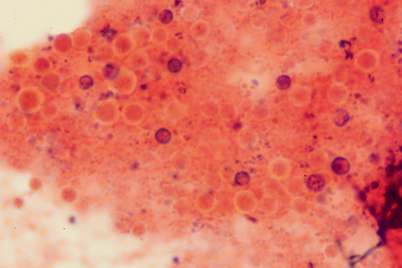

Fig. 15.6
Mouse tissues stained with various stains used to identify cryptococcal infection. Upper left panel is of brain stained with H&E showing meningoencephalitis with encapsulated yeast cells of Cryptococcus neoformans. The upper right panel is of kidney stained with GMS. The middle left panel demonstrates lung stained with Mayer’s mucicarmine. Note orange-red staining of polysaccharide capsular material of C. neoformans. The middle right panel is liver tissue stained with PAS. Lung stained with Alcian blue stain is seen in the bottom left panel. Lung stained by Fontana-Masson method is seen in the bottom right. Melanin pigment in the cell wall of C. neoformans stains dark with this stain. GMS Gomori methenamine silver, H&E hematoxylin and eosin, PAS periodic acid-Schiff. (Courtesy of Dr. W. A. Schell)

Fig. 15.7
Gram stain of sputum of a patient with pulmonary cryptococcosis. Cryptococcus neoformans appears as poorly stained gram-positive budding yeasts. (Courtesy of Dr. W. A. Schell, Duke University Medical Center)
Serology
Diagnosis of cryptococcosis has improved significantly over the past several decades with the development of serological tests for cryptococcal polysaccharide antigen and/or antibody. Use of serum cryptococcal antibodies for diagnosis of cryptococcosis has not been adopted. In contrast, detection of cryptococcal capsular polysaccharide antigen in serum or body fluids by a latex agglutination (LA) technique has been robust in its performance and is considered the gold standard diagnostic test for serological diagnosis of cryptococcosis. This test uses latex particles coated with polyclonal cryptococcal capsular antibodies or anti-GXM monoclonal antibodies and has overall sensitivities and specificities of 93–100 % and 93–98 %, respectively [28, 29]. The false-positive rate of cryptococcal capsular polysaccharide antigen testing is 0–0.4 % [30]. The majority of false-positive results can be explained by technical error (improper boiling/treatment), presence of rheumatoid factor or interference proteins, and infections with Trichosporon beigelii [31] or some bacterial species [32]. However, most of the false-positive results of LA testing for cryptococcal polysaccharide antigen have initial reciprocal titers of 8 or less [28]. Therefore, results of such low titers must be carefully interpreted within the clinical context. False-negative results of the LA test for cryptococcal polysaccharide antigen in cryptococcal meningitis are unusual but can be seen due to a prozone effect, and, therefore, high-risk negative specimens should be diluted and retested [33]. Low fungal burden, as in chronic low-grade cryptococcal meningitis or in the very early stages of cryptococcal infection, and improper storage of patient sera can also cause false-negative results in LA cryptococcal polysaccharide antigen tests [34].
Enzyme immunoassays (EIAs) for detection and quantification of cryptococcal polysaccharide antigen of all four serotypes of C. neoformans in sera and CSF have been developed to detect the major component of the polysaccharide capsule, GXM, with sensitivities and specificities of 85.2–99 and 97 %, respectively [28, 35]. This methodology is automated and overcomes some of the practical limitations of LA testing. Previous studies have compared EIA and LA assays and revealed no significant difference between these testing methods. EIA for cryptococcal polysaccharide antigen does not give discrepant results with rheumatoid factor or serum macroglobulins and is not affected by prozone reactions. Both LA and EIA testing have been rigorously studied and are recommended for use in both serum and CSF samples.
Recently, a lateral flow assay (LFA) was introduced in the diagnostic repertoire for cryptococcal infection and is Food and Drug Administration (FDA) approved for use in serum and CSF. The semiquantitative LFA offers many advantages over other serological methods, including rapid turnaround (approximately 15 minutes), minimal requirements for specialized laboratory infrastructure, stability at room temperature, and low cost [36]. The LFA has been evaluated against both EIA and culture, with sensitivities of 96–100 % for serum and plasma and 70–94 % for urine samples [36–39]. This assay has good performance across a broad range of clinical settings, including resource-limited settings and among cohorts with low burden of HIV infection and high rates of C. gattii infection, for which some EIA and LA tests are known to be insensitive [36–40]. The satisfactory performance of LFA combined with established cost-effectiveness and practical advantages of this approach support its use as a point-of-care testing (including preemptive screening of high-risk patients) in resource-limited settings [36, 37, 41].
Although the presence of cryptococcal polysaccharide antigen in serum is undoubtedly suggestive for dissemination of cryptococcal infection outside the lung, the precise value of cryptococcal polysaccharide antigen for diagnosis of nondisseminated pulmonary cryptococcosis remains less certain. Generally speaking, detectable cryptococcal antigen in serum should make clinicians consider that infection is now also located outside the lung. In a high-risk patient with clinical symptoms suggestive of meningitis, identification of cryptococcal antigen in CSF or serum is rapid, specific, noninvasive, and virtually diagnostic of meningoencephalitis or disseminated cryptococcosis even when the India ink examination or culture is negative [42, 43]. The LA test for serum cryptococcal polysaccharide antigen is widely used for detecting cryptococcal infection in patients with AIDS, as an initial screening test for those with fever of unclear etiologies or neurological symptoms. In some patients, it may represent the only means of achieving an etiologic diagnosis of invasive cryptococcosis or early diagnosis prior to CNS involvement.
Likely because of its sensitivity, the detection of cryptococcal polysaccharide antigen in the serum may precede clinically obvious disseminated cryptococcal disease (“isolated cryptococcal polysaccharidemia”) in severely immunosuppressed patients [44–46]. The management of these cases, in which there is a positive serum antigen and other nonspecific clinical findings in HIV-infected patients with negative fluid or tissue cultures, is uncertain. Persons of high risk with isolated cryptococcal antigenemia probably do benefit from antifungal therapy to prevent or delay the development of overt cryptococcosis [44]. Generally, positive serum antigen tests at titers of 1:4 or more strongly suggest cryptococcal infections in these patients.
Baseline cryptococcal polysaccharide antigen titers in serum and CSF may carry prognostic significance in patients with cryptococcal meningitis [47]. A study in HIV-related acute cryptococcal meningitis indicated that a baseline titer of CSF cryptococcal polysaccharide antigen of 1:1024 or greater was a predictor of death during systemic antifungal treatment [48]. After initiation of systemic antifungal therapy, patients may respond to treatment and titers of cryptococcal polysaccharide antigen fall. Similarly, a rise in CSF cryptococcal polysaccharide antigen titers during suppressive therapy has been associated with relapse [49]. However, it is important to emphasize that the use of changing antigen titers to make therapeutic decision should be done with caution, as titers may not be equivalent across different serological modalities [39]. The kinetics of polysaccharide elimination remains unclear and, despite the accuracy of commercial kits for general diagnosis, the accuracy of specific titers can vary from kit to kit even from the same clinical specimen.
Culture and Identification
Cryptococcus can be easily grown from biologic samples such as CSF, sputum, and skin biopsy on routine fungal and bacterial culture media. Colonies can usually be observed on solid agar plates after 48–72 h of incubation at 30–35 °C in aerobic conditions. Antibacterial agents, preferably chloramphenicol, can be added to the media when bacterial contamination is considered. The yeast, however, do not grow in the presence of cycloheximide at the concentration used in selective fungal isolation media (25 µg/mL). Despite relatively rapid growth for most strains, cultures should be held for 3–4 weeks before discarding, particularly for patients already receiving antifungal treatment. Conversely, cultures may be negative despite positive microscopic examinations (India ink) due to nonviable yeast cells, which may persist for a prolonged period of time at the site of infection. Positive blood cultures are frequently reported in AIDS patients and may actually be the first positive test for cryptococcal infection in a febrile high-risk patient.
C. neoformans colonies will appear on routine fungal media as opaque, white, creamy colonies that may turn orange-tan or brown after prolonged incubation. The mucoid appearance of the colony is related to the capsule size around the yeasts. Cryptococcus does not routinely produce hyphae or pseudohyphae, or ferment sugars, but is able to assimilate inositol and hydrolyze urea [50]. C. neoformans and C. gattii have the ability to use galactose, maltose, galactitol, and sucrose [50]. There are special media such as canavanine-glycine-bromthymol blue (CGB) agar that can be used to differentiate C. gattii strains from C. neoformans strains [51].
Molecular Identification Methods
A number of molecular techniques have been developed for identification of cryptococcal species from biological specimens including single and multiplex polymerase chain reaction (PCR) fingerprinting, random amplified polymorphic DNA (RAPD), PCR restriction fragment length polymorphism (RFLP) analysis, multi-locus sequence typing (MLST), and matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry [52–58]. These highly sensitive and specific methods have been evaluated with a variety of biologic samples [59] and can rapidly identify to the species and subspecies/genotypic level, including identification of recognized and novel strains within geographical niches [60]. While the expense and specialized techniques required of these methods preclude widespread use in clinical practice, their use in larger-scale investigations will continue to enhance our understanding of the epidemiology, pathogenesis, and nuances of antifungal management, as well as identify microevolution of different strains [61].
Treatment
The Infectious Diseases Society of America Clinical Practice Guidelines for the Management of Cryptococcal Disease (summarized in Tables 15.5, 15.6), updated in 2010, provide a suitable framework for therapeutic decision making [62]. The updated guidelines provide detailed recommendations for specific “at-risk” populations and address different management strategies based on host, site of infection, and potential complications of cryptococcal infection. While subtle nuances exist based on host and site of infection, general principles for the management of cryptococcal infection can provide the cornerstone of a treatment plan in most cases.
Table 15.5
Treatment recommendations for cryptococcal meningoencephalitis. (Adapted from the 2010 IDSA Clinical Practice Guideline for the Management of Cryptococcal Disease with personal suggestions [62])
Human immunodeficiency virus-infected individuals a | |
|---|---|
Induction therapy: | |
Primary regimen | |
AmBd (0.7–1 mg/kg/day) plus flucytosine (5-FC; 100 mg/kg/day) | 2 weeks |
Liposomal AmB (3–4 mg/kg/day) or AmB lipid complex (ABLC; 5 mg/kg/day) | |
pls 5-FC(100 mg/kg/day) for patients predisposed to renal dysfunction | 2 weeks |
Alternative regimens b | 4–6 weeks |
AmBd (0.7–1 mg/kg/day) or liposomal AmBc (3–4 mg/kg/day) or ABLC (5 mg/kg/day) if flucytosine-intolerant | 2 weeks |
AmBd (0.7–1 mg/kg/day) plus fluconazole (800 mg/day) | 6 weeks |
Fluconazole (> 800 mg/day, preferably 1200 mg/day) plus 5-FC (100 mg/kg/day) | 10–12 weeks |
Fluconazole (800–2000 mg/day, preferably 1200 mg/day) | 10–12 weeks |
Itraconazole (200 mg BID) | 8 weeks |
Consolidation therapy: fluconazole (400 mg/day) | > 1 yeard |
Maintenance or suppressive therapy: fluconazole (200 mg/day) | > 1 yeard |
Alternative regimens b | |
Itraconazole (200 mg BID) AmBd (1 mg/kg IV per week) | > 1 yeard |
Organ Transplant Recipients e | |
Induction therapy: | 2 weeks |
Primary regimen | |
Liposomal AmB (3–4 mg/kg/day) or ABLC (5 mg/kg/day) plus 5-FC (100 mg/kg/day) | 4–6 weeks |
Alternative regimen (if flucytosine-intolerant) | |
Liposomal AmBc (up to 6 mg/kg/day) or ABLC (5 mg/kg/day) AmBd (0.7 mg/kg/day)g | 4–6 weeks |
Consolidation therapy: fluconazole (400–800 mg/day)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|