Fig. 7.1
Rathke’s pouch projects from the roof of the stomodeum and grows toward the infundibulum during the fourth week of gestation. During the sixth week of gestation, the connection between Rathke’s pouch and the pharyngohypophyseal stalk disappears. Rathke’s pouch then develops into the adenohypophysis. Craniopharyngiomas are generally believed to develop from squamous cell rests along the path of the primitive craniopharyngeal duct and adenohypophysis
7.3.2 Classification
Although the adamantinomatous type is common in all age groups, the squamous papillary type is rare in children. In pediatric studies, 92–96 % were adamantinomatous, 0 % were squamous papillary, 0–4 % were mixed, and 4 % were not classified (Miller 1994; Weiner et al. 1994). In adults, 63–66 % were adamantinomatous, 27–28 % were squamous papillary, 6–7 % were mixed, and 3 % were not classified. The squamous papillary tumors are seen almost exclusively in adults and make up 11–14 % of all craniopharyngiomas and approximately 28 % of adult tumors (Weiner et al. 1994; Miller 1994; Pekmezci et al. 2010).
Aside from the pathologic subgroups, craniopharyngiomas can be divided into four groups based upon their relationship to the optic chiasm. These include sellar or infrachiasmatic, prechiasmatic, retrochiasmatic, and giant or laterally expansile tumors. This classification is important in understanding how tumors in each of these locations present and, as will later be discussed, the different operative approaches and considerations that must be taken into account for each anatomic location. Sellar or subchiasmatic tumors do not typically involve the optic chiasm and, therefore, do not present with visual disturbances. They can, however, present with pituitary dysfunction as invasion into the pituitary gland can occur when the diaphragma is not preserved. Prechiasmatic tumors grow between the optic nerves and tend to expand anteriorly. Thus, they typically avoid the development of hydrocephalus but can lead to visual deficits from compression of the optic nerve(s) or the chiasm. Retrochiasmatic lesions tend to push the chiasm anteriorly and can thin the chiasm. These tumors often grow into and fill the third ventricle, which leads to the development of hydrocephalus and patients may present with signs and/or symptoms of increased intracranial pressure. Giant tumors can present with any of the above findings including visual or endocrine disturbance, signs of elevated intracranial pressure, or even posterior fossa signs (Hoffman 1994).
7.3.3 Histopathology
The adamantinomatous subtype is characterized by cysts, which contain a cholesterol rich, brownish color fluid, along with clusters of columnar cells and loose stellate zones (Sidawy and Jannotta 1997). Pale eosinophilic masses, known as “wet keratin” or “keratin nodules,” can be seen from desquamated epithelium, and areas of calcification are common. The tissue is very similar to tumors of tooth-forming tissues seen in the oropharynx and long bones which are called adamantinomas, hence the term adamantinomatous craniopharyngiomas. Microscopically, the epithelium consists of a basal layer of small basophilic cells, followed by an intermediate layer of variable thickness composed of a loose collection of stellate cells whose processes traverse the intercellular spaces (Fig. 7.2). The top layer consists of keratinized squamous cells, which desquamate as stacks of flat keratin plates within the cyst cavity. Therefore, the cyst fluid is rich in membrane lipids such as cholesterol and keratin and can cause chronic inflammation within the cyst walls. The desquamated cells often calcify and can rarely progress to metaplastic bone formation (Miller 1994). Areas of gliosis with Rosenthal fibers are also frequently seen at the interface between tumor and surrounding normal tissue. The papillary subtype consists of well-differentiated stratified, squamous epithelium organized into cords that extend into the surrounding tissues. Typically, calcifications and desquamated cells are absent (Muller 2014). The cyst fluid is typically lighter in color, and the cells are more tightly arranged in comparison (Miller 1994; Prabhu and Brown 2005). Mixed craniopharyngiomas do exist and have features of both adamantinomatous and papillary types (Prieto and Pascual 2013).
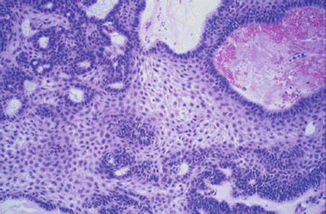

Fig. 7.2
Adamantinomatous craniopharyngioma showing palisading basal squamous epithelium surrounding loosely arranged epithelial cells (stellate reticulum) and nodules of eosinophilic keratinized cells
7.3.4 Tumor Biology
The oncogenesis of craniopharyngioma remains unclear. Although a number of previously identified markers used in the characterization of other tumors have been used, their value for these tumors is limited. For example, although the proliferative activity of craniopharyngiomas based on their MIB-1 immunostaining for the Ki-67 nuclear antigen is known, no correlation was identified with morphological features or clinical outcomes (Raghavan et al. 2000). The estrogen receptor gene is expressed in the proliferative epithelial component of adamantinomatous and papillary craniopharyngiomas, suggesting hormonal involvement in the genesis and/or progression of craniopharyngiomas, but no correlation was identified with clinical outcome (Thapar et al. 1994). Strong cytoplasmic immunoreactivity for vascular endothelial growth factor (VEGF) in the epithelial cells of both adamantinomatous and papillary craniopharyngiomas was identified and microvessel density, a measure of angiogenesis, correlated with an increased risk of recurrence (Vidal et al. 2002). However, not every recurrent tumor had a high microvessel density, indicating that other factors are involved.
The genetic alterations involved in the pathogenesis of craniopharyngiomas are not well known. Sarubi et al. studied three genes associated with odontogenic tumors, Gsα, Gi2α, and patched (PTCH), in a group of 22 adamantinomatous craniopharyngiomas but did not identify any mutations (Sarubi et al. 2001). Matsuo et al. demonstrated the expression of prostaglandin H synthetase-2 (PHS-2) in a variety of brain tumors, including two out of four craniopharyngiomas, but the significance of this isolated finding remains unclear (Matsuo et al. 2001). Nozaki et al. found no evidence of TP53 mutations in four craniopharyngiomas (Nozaki et al. 1998).
Buslei et al. examined the role of the Wnt signaling pathway in the pathogenesis of craniopharyngioma. Nuclear localization of beta-catenin, a transcriptional regulator involved in tumorigenesis and inhibited by the Wnt signaling cascade (Takamaru et al. 2008), is seen in adamantinomatous craniopharyngioma, but not in the more benign Rathke’s cleft cyst (Hoffman et al. 2006). This group also found mutations within exon 3 of the CTNNB1 gene, which codes for beta-catenin and translates into aberrant target gene expression within the adamantinomatous craniopharyngioma (Hölsken et al. 2008). Although intriguing, it is not clear if new therapeutic agents can be developed to take advantage of these targets.
7.4 Clinical Features
7.4.1 Neurologic Signs and Symptoms
The anatomic location of craniopharyngiomas in the sellar and suprasellar region results in predictable clinical patterns. Symptoms and signs develop due to compression or destruction of the optic chiasm, nerves and/or tracts, hypothalamus, pituitary stalk, or adjacent vascular structures. Children typically present with headache (50–70 %), visual disturbance (23–58 %), or endocrine abnormalities (10–33 %) (de Vries et al. 2003; Merchant et al. 2002; Stripp et al. 2004). Mental status changes are unusual in children, but occur in 25 % of adults.
The exact location of the tumor can result in varying clinical pictures. For example, retrochiasmatic tumors will displace the chiasm in an anterior direction and grow into the third ventricle leading to hydrocephalus; thus, roughly 60 % of patients present with headache, 50 % with nausea, 35 % with vomiting, and 10–20 % with lethargy (Sanford 1994; Hoffman et al. 1999; Merchant et al. 2002; de Vries et al. 2003; Stripp et al. 2004). A midline suprasellar mass typically causes a superior temporal quadrantanopia by compression of the overlying optic chiasm, but eccentric growth of a craniopharyngioma can lead to patterns of visual loss that vary in type and severity. Eighty percent of adults experience visual disturbance, while only 20–63 % of children have this sign (Merchant et al. 2002; de Vries et al. 2003; Stripp et al. 2004); this discrepancy may be due to the lack of awareness among children of a progressive narrowing of the peripheral fields. Toddlers, in particular, can become virtually blind before the extent of visual loss becomes apparent. Visual acuity and field testing should be performed in all patients, although accurate field testing is difficult to perform in young children.
7.4.2 Endocrine Signs and Symptoms
Craniopharyngiomas can compress or destroy the hypothalamus, anterior pituitary, or the pituitary stalk, leading to varying types of endocrinopathy. Virtually all of the pituitary hormones can be affected, including GH (75 %), luteinizing hormone (LH) or follicle-stimulating hormone (FSH) (40–44 %), adrenocorticotropic hormone (ACTH) (25–56 %), and thyroid-stimulating hormone (TSH) (25–64 %). Hyperprolactinemia occurs in 1–20 % of cases from impingement on the pituitary stalk (also known as the “stalk effect”), due to reduced amounts of prolactin inhibitory factor (mainly dopamine) reaching the lactotrophs of the anterior pituitary. Diabetes insipidus occurs only in approximately 16 % of patients prior to surgery (Sanford and Muhlbauer 1991; Honegger et al. 1999; Moore and Couldwell 2000; de Vries et al. 2003), although it is extremely common in the postoperative setting (see Sect. 7.9.1).
GH deficiency, hypothyroidism, and gonadotropin deficiency are the three most common endocrine abnormalities at presentation in children (Sanford and Muhlbauer 1991; Merchant et al. 2002; de Vries et al. 2003; Stripp et al. 2004). Short stature is the most common endocrinologic aberration on presentation occurring in about 33 % of pediatric cases (Merchant et al. 2002). However, review of the German Kraniopharyngiom database reveals that virtually all children exhibit a reduction in growth prior to diagnosis (Muller et al. 2004).
Hypothyroidism leads to poor growth, weight gain, cold intolerance, and fatigability (Rose et al. 1999b; Zhou and Shi 2004). Gonadotropin deficiency may only be evident in adolescents, but interferes with the pubertal growth spurt. Growth failure can be a result of GH deficiency, central hypothyroidism, gonadotropin deficiency, or a combination of all three. ACTH deficiency is less common at presentation (Honegger et al. 1999), but is potentially life-threatening (see Sect. 7.9.4). Lastly, many of these children have increased body mass index (BMI) at presentation, due to continued weight gain in the absence of normal growth (Muller et al. 2004). However, the obesity is likely to worsen, due to post-therapy damage of the ventromedial hypothalamus, with resultant dysregulation of energy balance, termed “hypothalamic obesity” (see Sect. 7.9.4) (Hoffman et al. 1999; Lustig 2002, 2008; Lustig et al. 2003).
7.5 Natural History
Craniopharyngiomas are histologically and cytologically benign, but locally aggressive and tend to recur. Untreated craniopharyngiomas demonstrate progressive growth causing mass effect or hydrocephalus. The rate of recurrence with any form of treatment is 8–26 % at 5 years and 9–100 % at 10 years (Fahlbusch et al. 1999; Stripp et al. 2004). If recurrence cannot be controlled, local invasion and growth can result in death. Malignant change, however, is extremely rare. There is one report of an adamantinomatous craniopharyngioma in a patient who underwent surgical resections and three courses of radiotherapy, which underwent subsequent transition into a moderately differentiated squamous cell carcinoma (Kristopaitis et al. 2000). Other cases of malignant transformation reported in literature were presumably from transplantation of tumor fragments during surgery or from meningeal seeding (Barloon et al. 1988; Ragoowansi and Piepgras 1991; Malik et al. 1992; Israel and Pomeranz 1995; Gupta et al. 1999; Lee et al. 1999; Ito et al. 2001).
7.6 Diagnosis and Imaging
7.6.1 Computed Tomography and Magnetic Resonance Imaging
While plain films of the skull are rarely used today, they can provide useful diagnostic information that may suggest a craniopharyngioma. Up to 65 % of adults and 90 % of children will have abnormal findings on plain films such as erosion of the sella, enlargement of the sella, or calcification in the location of the tumor (Harwood Nash 1994). Adult tumors have associated calcification approximately 40 % of the time, compared to 85 % of pediatric cases (Moore and Couldwell 2000). Sellar enlargement is seen in 65 % of patients, while sellar erosion is seen in 44 % (Donovan and Nesbit 1996).
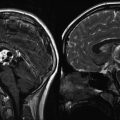
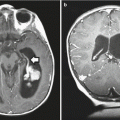
CT and MRI are much more commonly used today as initial diagnostic imaging tools. Typically, CT will reveal a mixed density, often lobulated, lesion consisting of solid and cystic portions, with hyperdense calcifications frequently present (Fig. 7.3). Any associated hydrocephalus or erosion of the sella can also be clearly evaluated by CT. The cystic component will appear iso- or hypodense. Most frequently, tumors will have both an intrasellar and suprasellar component (75 %), while a smaller proportion will be purely suprasellar (20 %) and very rarely can be located purely within the third ventricle.
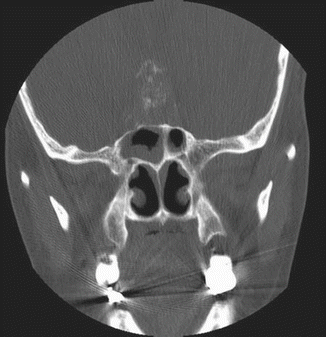

Fig. 7.3
CT scan (coronal view) showing punctate calcifications within a tumor
An MRI with and without contrast administration is the study of choice for craniopharyngioma. While calcification can be more difficult to appreciate, an MRI defines the critical relationships between the tumor and the surrounding vessels, optic chiasm, hypothalamus, and sella. Similar to CT, the tumor will have a heterogeneous appearance with the cystic portion of adamantinomatous lesions appearing iso- or even hyperintense on T1-weighted images due to the high protein content within the cyst. If a papillary tumor has a cystic component, the cyst more frequently resembles CSF density. The solid component, of either histologic subtype, will avidly enhance after the administration of contrast (Figs. 7.4 and 7.5).
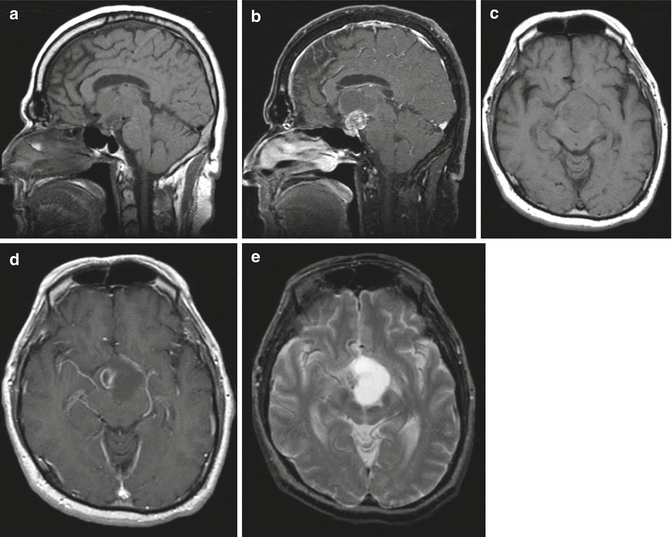
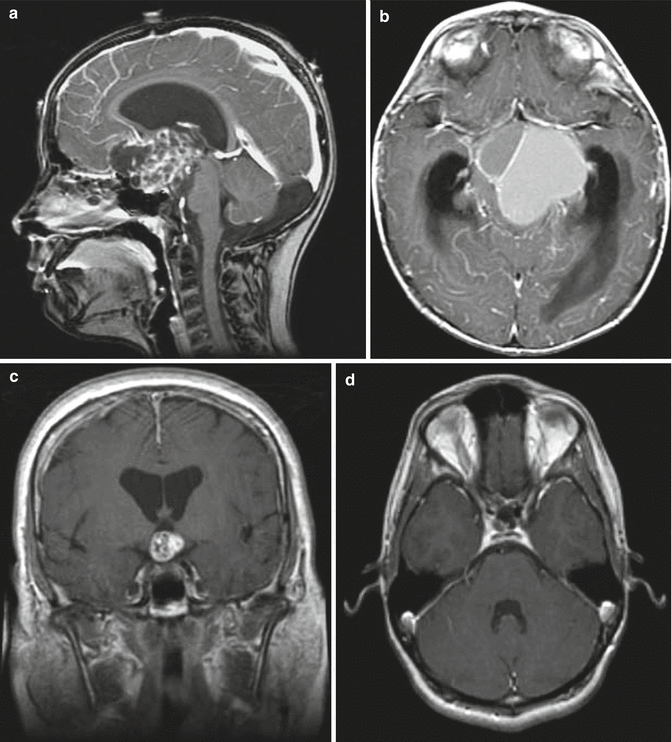

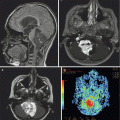
Fig. 7.4
Multiple MRI sequences of a typical mixed solid and cystic craniopharyngioma: (a) Sagittal T1-weighted image without contrast. A multilobulated mass is seen in the suprasellar region. (b) Sagittal T1-weighted image following gadolinium. The suprasellar solid component enhances, while the cystic area above it does not. (c) Axial T1-weighted image without contrast. (d) Axial T1-weighted image with gadolinium, (e) axial T2-weighted image

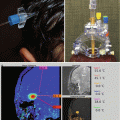
Fig. 7.5
Other examples of craniopharyngiomas: (a) Sagittal T1-weighted image with contrast showing a large mixed solid and cystic craniopharyngioma. (b) Axial T1-weighted image with contrast showing a large cystic craniopharyngioma with two major compartments. (c) Coronal T1-weighted image with contrast showing a small, solid suprasellar craniopharyngioma. (d) Axial T1-weighted image with contrast showing a small, recurrent craniopharyngioma within the sella
The differential diagnosis of cystic suprasellar masses in children includes Rathke’s cleft cysts (which usually do not have a solid component, are not lobulated, are nonenhancing, and are more homogeneous), pituitary adenomas (which enlarge the sella, are more homogeneous, and are usually less cystic), meningiomas (which are rarely cystic and are isointense on T1- and T2-weighted images), optic pathway gliomas (which are usually not calcified), and giant aneurysms (which usually contain a laminated thrombus) (Donovan and Nesbit 1996; Fischbein et al. 2000).
7.6.2 Clinical Evaluation
The evaluation and management of patients with craniopharyngiomas requires a multidisciplinary team approach, with the active participation of subspecialties such as neurosurgery, radiation and medical oncology, neuroophthalmology, endocrinology, and psychology.
If the patient does not require immediate neurosurgical intervention, then they should have a complete assessment of visual acuity and a visual field examination prior to treatment. Endocrine function should be evaluated both clinically and by laboratory measurements. A complete endocrine assessment is necessary prior to surgery and is invaluable when varying degrees of endocrine dysfunction may develop (Table 7.1). Where possible, based on the projected time to surgery, hormonal deficiencies should be treated (Wilson et al. 1998). All patients should get stress-dose steroids prior to surgery on the assumption that normal ACTH regulation is blunted (Samii and Tatagiba 1997); however, the use of dexamethasone to reduce brain swelling provides more than adequate glucocorticoid coverage. Hypothyroidism can take several days to correct and should be begun preoperatively; however, thyroxine supplementation can induce hepatic P450 enzymes responsible for metabolizing glucocorticoid, thereby unmasking glucocorticoid insufficiency and leading to hypotension and shock. Thus, glucocorticoid must be replaced prior to thyroxine supplementation (Moore and Couldwell 2000). Finally, any fluid and electrolyte abnormalities, including diabetes insipidus, should be identified and treated prior to surgery.
Table 7.1
Endocrine evaluation
Endocrine function | Tests |
|---|---|
Adrenal axis | 8 a.m. cortisol level |
24-h urine free cortisol level | |
ACTH stimulation test | |
Metyrapone test (difficult to get metyrapone currently) | |
Thyroid axis | Free T4 level |
Thyroid-stimulating hormone level | |
Thyarotropin-releasing hormone stimulation test in questionable cases | |
Gonadal axis | Follicle-stimulating hormone level |
Luteinizing hormone level | |
Sex steroids: estradiol in women, testosterone in men | |
Growth hormone (GH) | IGF-I level |
IGFBP-3 level | |
GH stimulation test (GH is pulsatile and low during the day, so a single random level is useless) | |
Prolactin | Prolactin level |
Serum sodium or osmolality | |
Antidiuretic hormone (ADH) | Urine specific gravity or osmolality |
Fluid intake vs. urine output | |
Water deprivation test in difficult cases | |
Hypothalamic obesity | Oral glucose tolerance test with simultaneous insulin levels |
7.7 Treatment
7.7.1 Surgery
7.7.1.1 Surgical Indications
There are three goals in the surgical treatment of craniopharyngiomas: diagnosis, decompression, and prevention of recurrence (Van Effenterre and Boch 2002). For the most part, current imaging studies provide a high degree of confidence in terms of diagnosis. Hydrocephalus can be treated acutely with either an external ventricular drain or a ventriculoperitoneal shunt prior to definitive surgery. Patients with craniopharyngiomas who present with acute visual deterioration or symptoms of elevated intracranial pressure from tumor-associated mass effect also require urgent surgical decompression. Since endocrine abnormalities such as hypothyroidism or diabetes insipidus may take several days to correct, a patient who is neurologically stable should have surgery performed electively after all endocrine abnormalities are controlled. Patients with large tumors will benefit from dexamethasone to reduce cerebral edema.
The goals of the surgical procedure should be clearly defined, as well as a recognition of potential risks associated with the size, location, and nature of the tumor. A small purely sellar tumor can be removed completely with postoperative morbidity restricted to endocrine dysfunction. A larger tumor that extends into the third ventricle with attachment to the hypothalamus may be associated with substantial morbidity if complete resection is planned. The consequences of complete anterior and posterior pituitary dysfunction also vary depending on the patient’s age and whether he/she has already completed puberty. The expectations of the postoperative complications should be clearly explained to the patient’s family prior to surgery.
7.7.1.2 Surgical Approaches
The surgical approach for craniopharyngiomas is largely dictated by tumor location, size, consistency, and anatomical relationships, as well as comfort of the surgeon with the particular approach. Techniques include both open and endoscopic approaches, or a combination of both may be utilized. Open microsurgical procedures include pterional, subfrontal, bifrontal interhemispheric, interhemispheric transcallosal, and transcortical transventricular approaches. Modifications to the pterional approach, such as the orbitofrontal and orbitozygomatic approach, may also be employed. Typically, the interhemispheric transcallosal and transcortical transventricular approaches are reserved for purely intraventricular tumors. As with all operative approaches, there are advantages and disadvantages to each route, but a detailed description of each operative approach will not be covered here.
The pterional route allows for access to both prechiasmatic and retrochiasmatic lesions and provides a lateral view. However, a significant amount of retraction may be necessary for larger tumors after dissection of the Sylvian fissure. Furthermore, lesions which extend superiorly far into the third ventricle may require a combined orbitozygomatic approach to allow for optimal visualization. Subfrontal approaches are best for prechiasmatic lesions and allow for early visualization of the optic nerves, internal carotid arteries, and lamina terminalis. Disadvantages include difficult access to the sella without the use of an angled endoscope, possible olfactory nerve injury, and potential entry into the frontal sinus requiring cranialization. In the bifrontal interhemispheric approach, a wider view is obtained, and one can gain access to large, retrochiasmatic lesions, but with the added potential cost of bilateral frontal lobe injury. Again, interhemispheric transcallosal and transcortical transventricular approaches are most often used when approaching purely intraventricular tumors or when a combined approach is needed.
Historically, the transsphenoidal route was used purely for tumors which were confined to the sella and below the diaphragm. More recently, the extended endoscopic-endonasal approach allows access to tumors in the suprasellar compartment. The advantages of this approach include direct access to the inferior portion of the tumor and visualization of important structures such as the chiasm and hypothalamus, avoidance of brain retraction associated with open approaches, and cosmesis/avoidance of craniotomy (Fernandez-Miranda et al. 2012). One series from Pittsburgh included 17 children with a mean follow-up time of 35.3 months. All patients were treated by an endoscopic-endonasal approach unless they had a purely intraventricular tumor. GTR was achieved in 52.9 % of patients. Postoperatively, no patients developed new visual deficits, but over 75 % had worsened or new pituitary dysfunction, 78 % developed DI, and 33 % had new onset obesity. CSF leak occurred in 11 %, and more significantly, 41.2 % of the pediatric cohort experienced tumor recurrence during follow-up, with an average of 19.6 months of progression-free survival (Koutourousiou et al. 2013). In another smaller series of seven patients treated by an endoscopic-endonasal approach for suprasellar craniopharyngiomas, GTR was achieved in all patients (Ali et al. 2013). Visual deficits improved in all patients, CSF leak occurred in 15 %, DI occurred in 100 %, and five of the seven had worsening or new anterior pituitary dysfunction. The endoscopic-endonasal approach appears to result in comparable outcomes as open procedures, but there are limitations to its use.
For intrasellar and monocystic craniopharyngiomas, the transsphenoidal approach is ideal in allowing drainage of the cyst and decompression of the optic chiasm. In a small group of cases at our institution, intracystic treatment with alcohol at the time of surgery resulted in excellent tumor control with minimal endocrine dysfunction (Fig. 7.6).
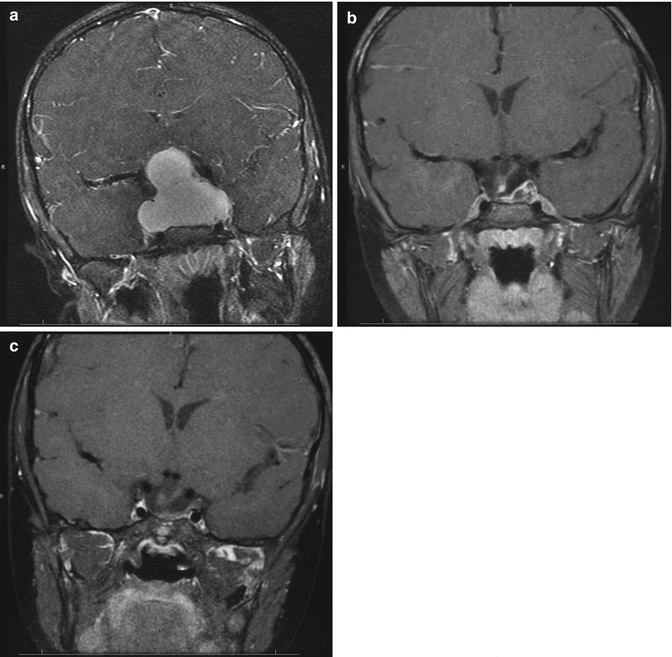

Fig. 7.6
(a) Coronal T1-weighted image with contrast showing a large cystic suprasellar craniopharyngioma in a 4-year-old girl. (b) Six months following a transsphenoidal procedure, the cyst is completely decompressed with a small residual lesion along the left side of the sella. This showed growth several years later and was removed by another transsphenoidal procedure. (c) Seven years after presentation, there is no residual tumor; the patient requires GH and thyroid supplementation but does not have DI
7.7.2 Radiation Therapy
7.7.2.1 Conventional Radiotherapy
Radiation has been a mainstay of craniopharyngioma treatment for decades and is utilized for both initial and salvage therapies for recurrences. While the benefit of radiation therapy is clear in terms of tumor control, it must be carefully balanced with the negative long-term consequences of radiation, particularly in very young children. Long-term side effects including secondary malignancies, neurocognitive decline, vasculopathy, endocrinologic disturbances, and visual disturbances have all been well described.
Conventional fractionated radiation therapy consists of the delivery of a high dose of radiation to a target by dividing the treatment into multiple doses, with a single dose, or fraction, given each day, allowing for normal tissue repair between fractions. Total doses have typically ranged from 50 to 65 Gy, divided into 1.8–2 Gy daily fractions (Merchant et al. 2002). Typically, the maximum dose delivered to the optic apparatus is 50–54 Gy. Current dosing is typically 50–54 Gy divided into daily fractions of 1.8–2 Gy. Cyst growth during or after radiation therapy has been well documented and requires frequent imaging to ensure appropriate volume modifications and interventions if indicated (Bishop et al. 2014; Merchant et al. 2013; Winkfield et al. 2009; Boehling).
7.7.2.2 Intensity-Modulated Radiotherapy
Intensity-modulated radiation therapy (IMRT), a form of photon therapy, utilizes “beamlets” of varying intensities in attempts to conform to the tumor boundaries with rapid falloff of dosage to surrounding tissues. This results in a lower dose to a larger area of surrounding tissue (integral dose) compared to conventional radiotherapy which results in a higher dose to smaller amount of surrounding tissue (Wisoff and Donahue 2015).
7.7.2.3 Stereotactic Radiosurgery
Stereotactic radiosurgery (SRS) or stereotactic radiotherapy (SRT) typically consists of one to five treatments, using either a Gamma Knife unit or a linear accelerator, of focused radiation to the target (tumor) and allows for a greater decrement in dosage to the surrounding normal tissues. No more than 8–10 Gy can be delivered in a single dose to the optic apparatus. Higher doses have been shown to result in optic neuropathy (Leber et al. 1998; Stafford et al. 2003). Therefore, multisession or fractionated treatment has become attractive for most craniopharyngioma patients given the tumor’s relationship to critical structures.
7.7.2.4 Fractionated Stereotactic Radiosurgery
Fractionated SRS is typically done in more than five, and usually 25–28, treatment sessions. This technique has the advantages of both fractionated external beam radiation therapy (normal tissue repair between fractions) and radiosurgery (minimal dose to structures away from the targeted region) and can be used to treat tumors that are greater than 3 cm, as well as those that abut the optic apparatus.
A large series from the University of Heidelberg (Combs et al. 2007) reported 100 % local control at both 5 and 10 years following treatment. Overall survival rates were 97 % and 89 %, respectively. The median target dose was 52.2 Gy, given with conventional fractionation of 1.8 Gy per fraction. A complete response was observed in 4 patients, partial response in 25 patients, and stable disease in 11 patients. There were no visual impairments or second malignancies reported at a median follow-up time of 98 months. These results were corroborated by a similar series using stereotactic techniques with conventional fractionation from the Royal Marsden Hospital (Minniti et al. 2007).
7.7.2.5 Proton Beam Therapy
Proton therapy is also being used for the treatment of craniopharyngioma patients. The benefit of proton therapy is the inverse dosing profile, which results in less radiation to normal tissues proximal to the target and nearly no radiation to normal tissues distal to the target as a product of the distal Bragg peak (Suit et al. 1975; Luu et al. 2006). This reduction in integral dosage to normal tissues has been quantified by Boehling et al. for a number of structures in comparison to IMRT including the hippocampus, brainstem, and vascular structures (Boehling et al. 2012). Theoretically, this may result in a decrease in radiation-induced vasculopathy and neurocognitive decline seen with other forms of radiotherapy. Future studies with long-term neurocognitive outcomes will help answer this question.
Recent publications have highlighted potential higher toxicities with protons as compared to photons. Using protons, the tumor volume and adjacent normal tissues receive high doses equivalent to those using photons, but dose-effect models were derived chiefly using photons, without incorporating uncertainties that still exist regarding proton-dose deposition and biological effects. A study examining imaging changes following proton- and photon-based (IMRT) radiotherapy demonstrated increased changes following proton-based radiation compared to photon-based IMRT. Furthermore, only proton-treated patients experienced grade 3 or 4 changes and had persistent symptoms, including one grade 5 toxicity related to radiation necrosis documented on autopsy (Gunther et al. 2015). A similar study assessing brainstem toxicity following proton radiotherapy for pediatric brain or skull-base tumors reported a 2-year cumulative incidence of grade 3 or higher brainstem toxicity of 2.1 ± 0.9 %, with one grade 5 toxicity (Indelicato et al. 2014).
The largest pediatric series using proton therapy includes a total of 16 patients aged 7–34 years (Luu et al. 2006). Patients were treated for both initial treatment following resection and for recurrences. Twelve patients survived, three of which developed treatment-related toxicity including panhypopituitarism at 36 months, cerebrovascular accident at 34 months, and meningioma in a patient who had previously received photon therapy. With a mean follow-up of 60 months, the overall tumor control rate was 93 %, which is comparable to other forms of radiation. A recent review comparing outcomes for proton therapy vs. conformal photon therapy found equivalent tumor control and a trend toward early cyst growth in the conformal photon therapy group that did not reach statistical significance (Bishop et al. 2014).
7.7.2.6 Intracavitary Radiation Therapy
Over 90 % of craniopharyngiomas have a cystic component. Frequently, the cyst is large and can comprise the majority of the tumor. Direct intracystic treatment has the goals of reducing cyst volume and achieving long-term tumor control. Leksell and Liden first described intracavitary therapy with the use of beta-emitting radionuclides such as 90yttrium, 32phosphorus, or 186rhenium into the cyst (Leksell 1952). From a review by Blackburn et al., 121 of 149 cysts treated in 127 patients reduced in size or were obliterated in the follow-up period of 0.2–13 years. However, the distinction between recurrence of a cyst and recollection of the initial lesion varied among the different studies (Blackburn et al. 1999). In a study of 30 patients treated with 32phosphorus, which cyst regression was defined as more than 50 % reduction in volume, 88 % of patients were found to have cyst regression, with response occurring within 3 months of surgery and continued decrease in cyst size up to 2 years after surgery (Pollock et al. 1995). Overall survival was 55 % at 5 years and 45 % at 10 years, with a mean survival of 9 years (Voges et al. 1997). The impact of intracavitary irradiation on vision varied widely among studies ranging from 100 % deterioration to 100 % improvement, with improvement in 53 % of patients, over all the studies considered (Voges et al. 1997; Blackburn et al. 1999). The major risks of intracavitary radiotherapy include visual loss and radiation necrosis of the hypothalamus. There are situations in which intracystic therapy is the only option, but its broad adoption has been limited by the difficulty in obtaining of these compounds and the process for handling/injecting.
7.7.3 Chemotherapy
7.7.3.1 Systemic Chemotherapy
There are no effective systemic chemotherapeutic agents for craniopharyngioma. The strongest evidence to date is for the use of interferon-alpha (IFN-α). Its use was based on its activity in squamous cell carcinoma and the observation that craniopharyngioma shares a similar epithelial cell origin. A phase II trial of IFN-α for progressive, recurrent, or unresectable craniopharyngiomas in children under 21 years of age was reported a number of years ago (Jakacki et al. 2000). Treatment consisted of an induction phase of 8,000,000 U/m2 daily for 16 weeks. Patients without progressive disease at 16 weeks then continued at the same dose three times a week for 32 weeks. Time to progression after discontinuation of IFN-α was 6–23 months. IFN-α toxicity occurred in 60 % of cases during the first 8 weeks of treatment, but resolved with discontinuation or dose reduction. Toxicities include hypoadrenal crisis with fever, neutropenia, transaminitis, fatigue, rash, insomnia, and seizures. A follow-up study of pegylated interferon-alpha 2b in a small group of children demonstrated activity of the agent with five out of five patients achieving radiographic responses (Yeung et al. 2012).
Insights into the genetics and molecular biology of craniopharyngioma may lead to new therapeutic opportunities. A subset of adamantinomatous craniopharyngiomas is of monoclonal origin (Rienstein et al. 2003; Sarubi et al. 2001). This suggests that an acquired somatic mutation may be an initiating event. Early studies also indicate very low levels of O-6 methylguanine DNA methyltransferase (MGMT) suggesting temozolomide may have a role in future treatment paradigms (Zuhur et al. 2011). Finally, in vitro testing of EGFR inhibitors has been successful in inhibiting tumor cell migration. Prior work has shown that EGFR is preferentially overexpressed at the border zone between tumor and normal brain tissue (Holsken et al. 2011).
Adamantinomatous craniopharyngiomas have also been shown to exhibit mutations in the beta-catenin gene CTNNB1 on chromosome 3 (Buslei et al. 2005; Cao et al. 2010; Kato et al. 2004). This mutation then leads changes in gene transcription that control angiogenesis, cell proliferation, and mobility. Of note, some craniopharyngioma cells have demonstrated increased levels of beta-catenin without mutations in CTNNB1 suggesting an alternative pathway for tumorigenesis. Future therapies directed at downregulating beta-catenin or inhibiting its interactions with certain transcription factors could prove to be quite beneficial in treating patients with adamantinomatous craniopharyngiomas.
A recently published mRNA microarray gene expression analysis of 15 patients with adamantinomatous craniopharyngioma identified a number of potentially actionable therapeutic targets in the transcriptome of these tumors. Of interest included frequent high expression of LCK, EPHA1, and SRC, which can all be targeted with the therapeutic agent dasatinib, as well as other targets of interest including SHH, MMP9, and MMP12 (Gump et al. 2015).
7.7.3.2 Intracavitary Chemotherapy
In 1985, Takahashi et al. reported their experience with intracystic bleomycin in seven pediatric patients as adjuvant therapy immediately following STR (Takahashi et al. 1985). Since that time, numerous reports have been published using intracystic bleomycin for both initial treatment and for tumor recurrences. The immediate side effects resulting from bleomycin treatment are generally mild and self-limited and include nausea, vomiting, and headache. In approximately 3 % of patients, more serious side effects occurred in a delayed fashion (Linnert and Gehl 2009). These include hearing loss, visual loss, hypothalamic dysfunction, cerebral ischemia, panhypopituitarism, and even death.
There are a number of limitations associated with the use of intracystic bleomycin. It has direct toxicity to CNS structures, and a number of steps must be taken to avoid any leakage into the subarachnoid space (Steinbok and Hukin 2010). The appropriate dose and frequency of injection have not been clearly defined. While initial tumor responses are promising, there are some results that suggest that all tumors will eventually grow despite treatment (Steinbok and Hukin 2010). In young children, this may be an acceptable option, since one goal may be to delay external radiation.
More recently IFN-α has also been used as an intracystic agent. In contrast to bleomycin, IFN-α is not toxic when placed in the subarachnoid space. Ierardi et al. suggested that IFN-α may reduce cyst volume by inducing apoptosis through activation of the Fas apoptotic pathway (Ierardi et al. 2007). In a series of 21 patients, all pediatric patients with adamantinomatous craniopharyngiomas, greater than 50 % of patients, had a complete response following treatment with intracystic IFN-α (Cavalheiro et al. 2010). An additional 30 % of patients had a partial response to treatment. Minor complications such as fatigue, weight loss, and behavioral changes were observed. The use of IFN-α in combination with other therapies may increase given its relatively benign side effect profile and lack of toxicity in comparison to bleomycin.
7.8 Outcome
On the basis of postoperative imaging, GTR varies widely in various series ranging from 29 % to 77 % of cases (Sanford 1994; Villani et al. 1997; Einhaus and Sanford 1999; Fahlbusch et al. 1999; Duff et al. 2000; Van Effenterre and Boch 2002; Stripp et al. 2004; Caldarelli et al. 2005; Shi et al. 2008). Reflecting the heterogeneity of patient groups, recurrence has been reported to occur in 8–100 % of patients after initial GTR. The mean duration of follow-up in these studies ranges from 5 to 10 years (Table 7.2) (Hetelekidis et al. 1993; Villani et al. 1997; Fahlbusch et al. 1999; Duff et al. 2000; Kalapurakal et al. 2000; Poretti et al. 2004; Stripp et al. 2004; Shi et al. 2008). Recurrence following GTR can be assumed to occur because of unrecognized deposits of tumor capsule. Even high-quality imaging will miss small amounts of epithelium that have the potential to develop into recurrent tumors. The use of adjunctive radiation therapy following GTR is controversial.
Table 7.2
Outcomes of primary surgery, gross total resection, for craniopharyngioma
References | Number of patients | Recurrence-free survival (%) | Percent survival |
|---|---|---|---|
Lin (2008) | 14 | 54 at 6 years | 100 at 6 years |
Shi (2008) | 276 | 86 at 6 years | 98 at 6 years |
Puget (2007) | 33 | 64 at 6 years | 94 at 6 years |
Bojanowski (2006)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|