Biopsy Techniques
Key Points
- •
Biopsy of suspicious breast lesions is necessary prior to considering operation.
Historical Context
Historically, biopsy of suspicious breast lesions required surgical excision. Lesions were sampled and sent for frozen section prior to performing a radical or modified radical mastectomy. It was not until the 1970s that percutaneous biopsy was introduced as an alternative. Image-guided biopsy was first described by Roberts et al. in 1975. However, it wasn’t widely utilized until 1993 following the success of ultrasound (US)-guided core needle biopsy (CNB).
Shorter recovery times, reduced cost, fewer complications, and smaller scars make percutaneous biopsy more favorable than surgical biopsy. Furthermore, the use of a minimally invasive biopsy technique to establish a breast cancer diagnosis before any surgical procedure is a Breast Quality Measure by the American Society of Breast Surgeons (ASBrS). For these reasons, image-guided percutaneous biopsies are preferable and commonly performed.
Indications
The Breast Imaging and Reporting Data Systems (BI-RADS) lexicon for mammography was established in 1993. BI-RADS describes key imaging findings and classifies breast lesions into different categories, which provides direction for management and follow-up. Lesions that are BI-RADS 4 or 5 on mammography require further evaluation with biopsy. US and magnetic resonance imaging (MRI) are other modalities with their own criteria to identify abnormal breast lesions requiring biopsy.
Fine Needle Aspiration Versus Core Needle Biopsy
For breast lesions, CNB is preferred over fine needle aspiration (FNA). CNB provides evaluation of tissue histology, while FNA only provides cytologic evaluation and cannot distinguish invasive from noninvasive cancer. Additionally, biomarker status is a crucial component of the breast cancer diagnosis and is difficult with FNA. FNA is associated with higher rates of insufficient samples and higher false-negative rates. However FNA is often able to provide a diagnosis when axillary lymph nodes are being sampled near a large vessel or when cystic lesions require evaluation.
Types of Needles and Devices
Both spring-loaded and vacuum-assisted devices are available. Vacuum-assisted devices have the advantage of allowing for a single insertion as well as larger tissue and broader tissue sampling secondary to a rotating device. Needle sizes typically range from 7 to 14 gauge and vary depending on the device type and by imaging modality. The number of samples obtained also vary by needle size, with smaller needles requiring more samples for increased accuracy.
Palpable Lesions Versus Nonpalpable Lesions
Breast lesions may be palpable or nonpalpable. If a lesion is palpable, image guidance with US is recommended. For lesions that are not palpable, stereotactic imaging, US, or MRI are necessary to identify and biopsy the lesion. The choice of modality depends on the ability to visualize the lesion as well as technical convenience. For instance, if a lesion is seen on both mammogram and US, US would be the preferred method of biopsy owing to ease and technical simplicity compared with stereotactic biopsy. For lesions demonstrated across multiple modalities, the radiologist must ensure that the lesions and targets are concordant between the images.
Considerations
As with all procedures, written informed consent is necessary prior to performing breast biopsy. Patients should be informed that biopsy may yield benign, malignant or nondiagnostic results. Pathologic results must be correlated with clinical findings and interpreted as concordant or discordant. Risks of the procedure include bleeding, infection, and missed lesions, and should be discussed. Anticoagulation is a relative contraindication to performing breast biopsy. For patients taking anticoagulant medications, it is usually recommended that patients hold their anticoagulation periprocedurally. Additionally, patients should be informed that a clip will be placed to mark the location of the biopsy. Regardless of the modality used or if the lesion is palpable or not, a clip should be placed at the biopsy site. In the event that biopsy results necessitate surgical intervention, the clip marks the site for surgery. If biopsy results are benign and concordant, the presence of a clip allows for continued surveillance and denotes that the lesion has been previously sampled.
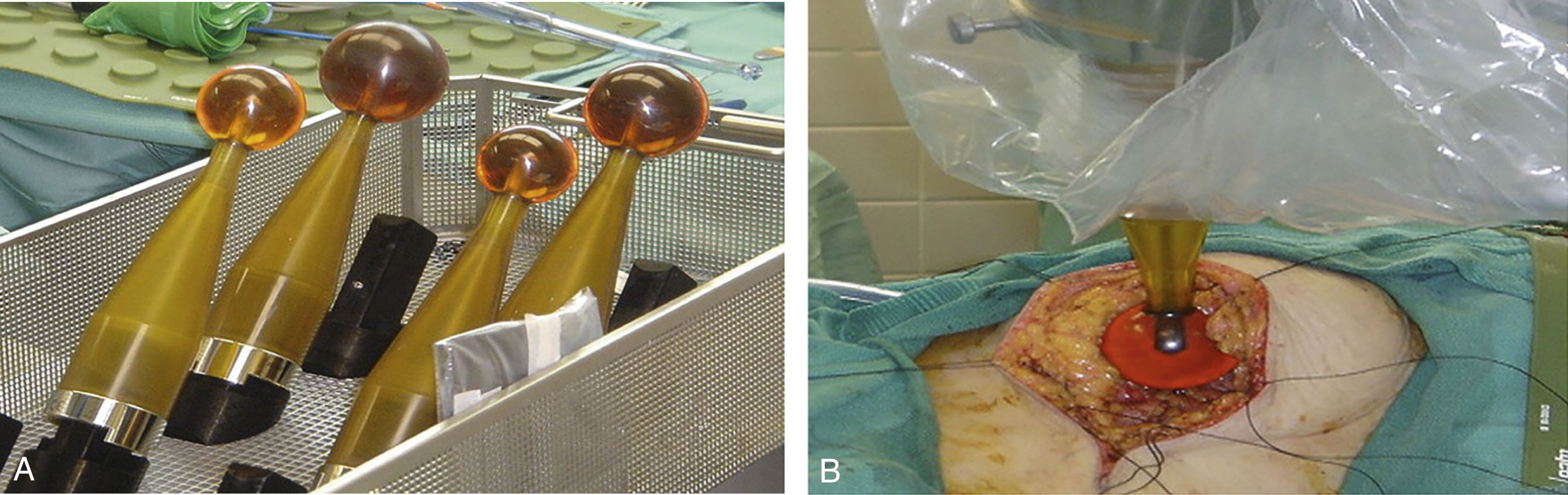
Stereotactic Biopsy
Stereotactic biopsy is the preferred modality for lesions that are mammographically detected without a sonographic correlate and can be performed via two-dimensional or three-dimensional mammographic guidance. Patients may be positioned prone or upright with advantages and disadvantages to each. In the prone position, the breast is placed through an opening in the table ( Fig. 7.1 ). It may be difficult for some patients to maneuver onto the table. In the upright position, the breast is compressed between two plates similar to the usual technique for mammography ( Fig. 7.2 ). In this position, the patient may experience vasovagal responses or move in response to seeing the needle. Upright tomosynthesis-guided vacuum-assisted breast biopsy has a higher rate of successful biopsy than prone and can be performed in a shorter amount of time with one-fourth of the exposure.
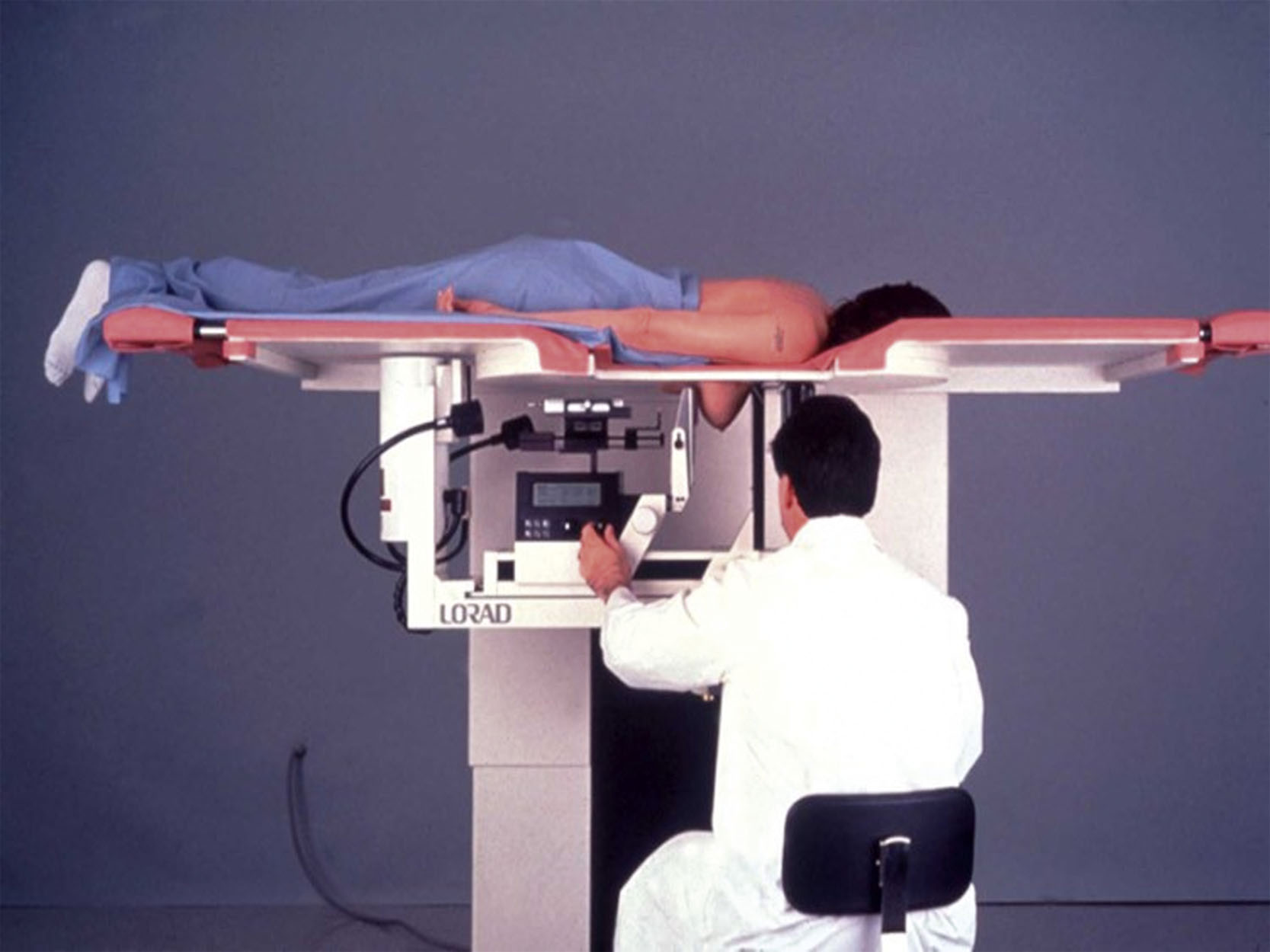
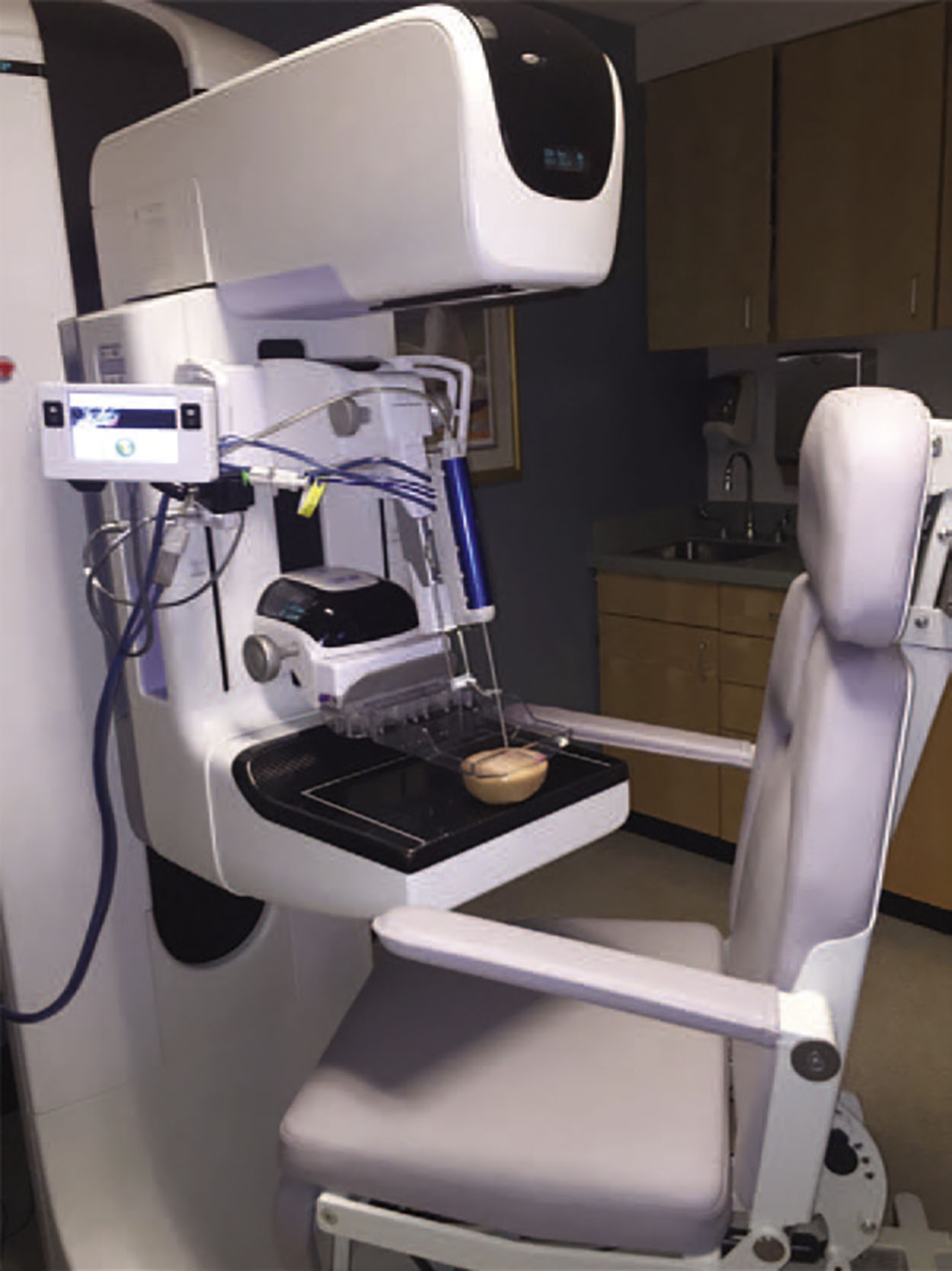
The breast is compressed and the target area positioned through the window in the paddle. A scout view is obtained. Prior to biopsy, it is necessary to ensure that there is adequate distance from the skin to the lesion so that the biopsy is within the breast tissue and not too superficial. Biopsies that are too superficial may excise skin as well as breast tissue. Local anesthetic is injected, biopsy performed, and a marking clip is placed. The number of samples depends on the size of the needle. For 10–11-gauge needles, typically 12 samples are obtained, while for 7–9-gauge needles, 4 samples are typical. A specimen radiograph is necessary to verify that the targeted lesion was biopsied. Manual compression is provided and the skin incision is reapproximated with steri-strip or surgical glue. A postprocedural mammogram is obtained to document accurate tissue sampling as well as to document marker placement. Biopsy clips have been known to migrate due to hematoma formation or upon release of breast compression, known as the accordion effect. Given its implications for future localization and surgical planning, clip migration must be documented in the biopsy report.
Ultrasound Biopsy
For sonographically detected lesions, US-guided biopsy is preferable to stereotactic biopsy ( Fig. 7.3 ). US-guided biopsy does not require breast compression, does not utilize ionizing radiation, and provides real-time visualization of the needle with shorter procedure times, lower costs, improved patient comfort, and is technically less challenging.
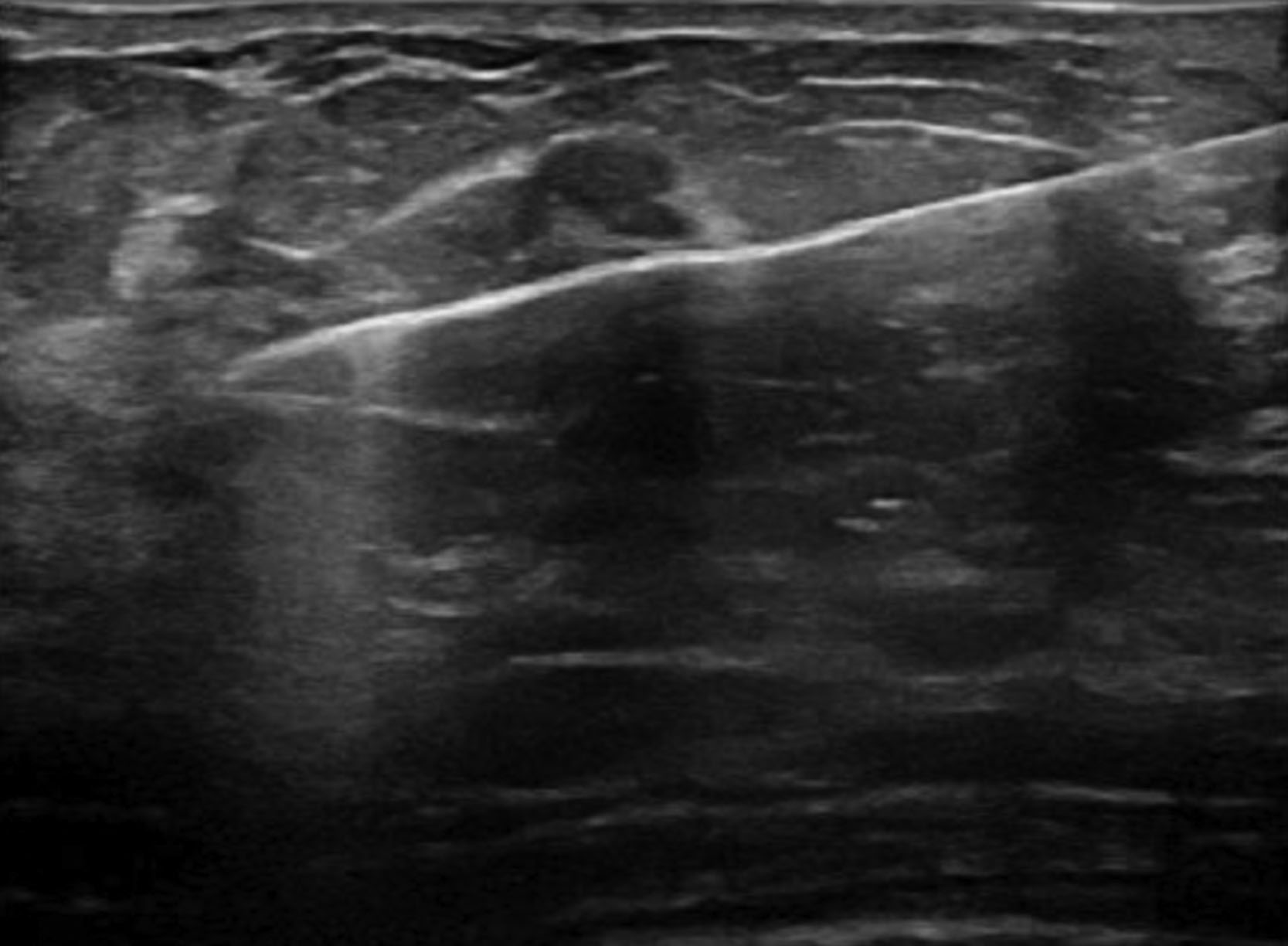
Typically, US is used for masses but can be utilized for calcifications. When calcifications or nonmass lesions are biopsied under US guidance, vacuum-assisted biopsy has been found to be more accurate than 14-gauge CNB. Furthermore, when calcifications are biopsied under US guidance, it is imperative to obtain postprocedural imaging. For US-guided biopsies, patients are positioned supine but may be bolstered in the lateral decubitus position if needed depending on the location of the lesion in the breast. Sometimes the ipsilateral arm is positioned overhead to facilitate biopsy. The US probe is held either by the radiologist or the technologist and is used to identify the lesion. After injection of local anesthetic, the skin is entered approximately 1–2 cm from the edge of the transducer and the needle is visualized throughout the entire procedure to ensure adequate tissue sampling and to avoid inadvertent injury. This is most easily achieved when the needle runs parallel to the transducer. An image is obtained as the needle traverses the lesion. As with stereotactic biopsy, a biopsy clip is placed followed by postprocedural imaging. Clip migration is less common with US-guided biopsies compared with stereotactic biopsies.
Magnetic Resonance Biopsy
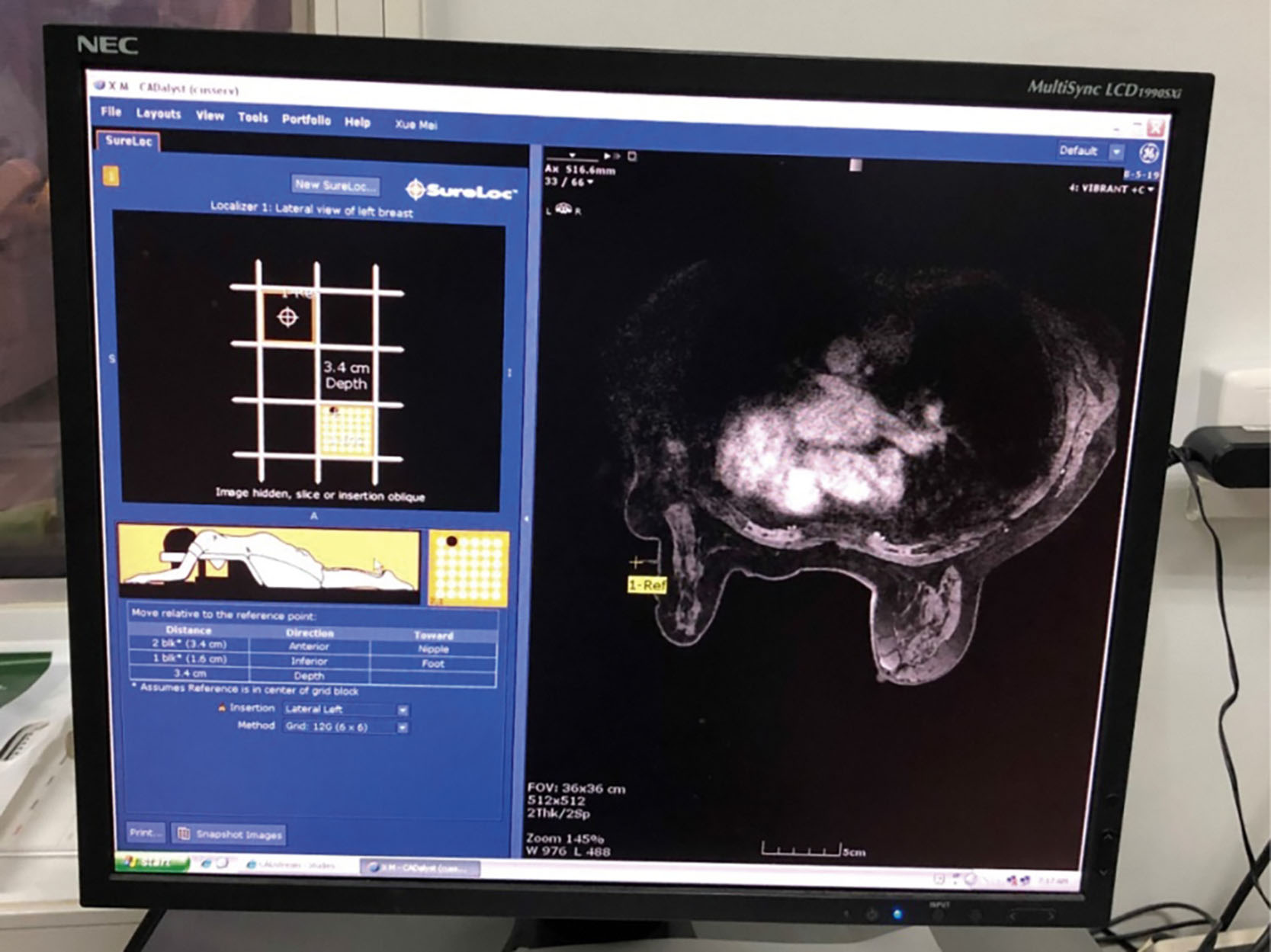
Magnetic resonance (MR)-guided biopsies are indicated for lesions detected with MR but without mammographic or US correlates. MR-guided biopsy is technically challenging, expensive, and time consuming. The patient is positioned prone with the breast positioned through an opening in the table with biopsy grid used to localize the lesion ( Fig. 7.4 ). Light compression maintains breast position while permitting vascular perfusion with contrast. Fluctuations in a patient’s hormones causing changes in tissue enhancement or breast compression occluding inflow of contrast material may result in lesion nonvisualization. If this occurs, short-term follow-up is recommended to ensure stability of lesions that are not visualized at the time of biopsy. If the lesion is identified, the needle is positioned using a grid and fired. Four to six samples from a 7–9-gauge needle are typically obtained. As with other modalities, a clip is routinely placed at the biopsy site.
Postbiopsy Considerations and Concordance
Following the procedure, a report is written describing the modality used, the lesion location and laterality, the number of tissue specimens obtained, clip placement, any associated complications, and results from the post-biopsy mammogram, including clip location. The procedure report is addended to reflect the pathology results and whether or not they are concordant with the clinical and imaging findings. Imaging-pathologic concordance must take into consideration several factors. Appropriate targeting of the lesion is essential. Stereotactic biopsy can be more easily monitored by postprocedural mammogram, while US-guided biopsies rely on real-time needle visualization. The biopsy specimen must also be adequate and requires sampling of the most suspicious area of the lesion.
Imaging-pathologic concordance is imperative to determine next steps for biopsied lesions. Benign concordant results should be followed in 6 months with the same modality used for biopsy in order to determine lesion stability. Suspicious lesions with benign pathology are considered discordant and require further intervention. Discordant results may either be rebiopsied or surgically excised.
Surgical Options
Key Points
- •
Trials have demonstrated the safety of surgical de-escalation for selected patients with breast cancer.
- •
It is necessary to consider patient age, clinical and imaging findings, and biomarker status when discussing surgical timing and options for patients with breast cancer.
- •
Treatment recommendations for breast cancer operations continue to evolve rapidly.
Historical Context
Breast cancer once relied on invasive, disfiguring operations for treatment. Today, a woman can often be cured with breast-conserving surgery and adjuvant therapies. Dr. Halsted pioneered the radical mastectomy in the late 1800s during which the entire breast, pectoralis major and minor muscles, and axillary lymph nodes were removed. It was believed that the tumor spread in a contiguous manner until the 1960s, when research demonstrated that breast cancer was often a systemic disease. Concurrent improvements in systemic therapy permitted a shift toward consideration of surgical de-escalation.
In the 1960s, the National Surgical Adjuvant Breast and Bowel Project (NSABP) was created by Dr. Bernard Fisher in order to collaborate on research and determine the best treatment for breast cancer. Two of the most influential studies from NSABP as they relate to breast cancer surgery include NSABP B-04 and NSABP B-06.
Between 1971 and 1974, NSABP B-04 randomized women with clinically node-negative disease to radical mastectomy, total mastectomy with radiation, or total mastectomy without radiation, and women with clinically node-positive disease to radical mastectomy or total mastectomy with radiation. Early results demonstrated no significant difference in disease-free survival, distant disease–free survival, and overall survival, and even after 25 years of follow-up no survival benefit was seen in any group. Interestingly, there was a statistically significant difference in locoregional recurrence among clinically node-negative women favoring those that underwent total mastectomy with radiation.
In NSABP B-06, patients were randomized to undergo modified radical mastectomy, lumpectomy with axillary dissection, or lumpectomy with axillary dissection plus whole-breast radiation. Once again, no difference in survival could be seen. However differences in local control were identified. Among women who underwent lumpectomy, the lowest ipsilateral breast tumor recurrence (IBTR) was seen in those who were treated with radiation.
These studies paved the way for further de-escalation of locoregional therapy. Between 1999 and 2004, NSABP B-32 demonstrated that sentinel node biopsy for clinically node-negative patients was as effective as standard axillary dissection with fewer side effects. Around the same time, the Alliance for Clinical Trials in Oncology Z0011 trial (ACOSOG Z0011) examined whether axillary dissection could be omitted for clinically node-negative women with axillary metastases identified by sentinel node biopsy undergoing lumpectomy and whole-breast radiation. Women in whom sentinel node biopsy identified one to two positive lymph nodes were randomized to undergo axillary dissection or no further surgery. No significant differences in disease-free survival, overall survival, or local or regional recurrences were seen between groups. ACOSOG Z0011 demonstrated that it was safe to omit axillary dissection in women with limited axillary disease treated with breast conservation. The study was criticized for closing early due to enrolling only 891 of the anticipated 1900 patients, as well as a lower than anticipated event rate. Study results stood the test of time and long-term follow-up continued to demonstrate no significant differences in outcomes.
Other studies including IBCSG 23-01 and the AMAROS trials also demonstrated no advantage to routine axillary dissection for women with minimal axillary disease. As a result, many clinically node-negative women with early-stage breast cancer can now be spared the morbidity of axillary dissection. Results from Z0011 were practice changing and led to changes in national guidelines.
Surgical Localization of the Biopsied Lesion
Following breast or axillary needle biopsy, it is imperative that a clip be placed in order to mark the location for future surgical excision. For women with nonpalpable lesions undergoing breast-conserving surgery, localization of the lesion is necessary. In the event that mastectomy is performed, localization is often not necessary, but may be considered for very superficial, deep, or lateral lesions. Localization can be performed via a number of different modalities.
Wire localization of breast lesions has been utilized since the 1970s ( Fig. 7.5 ). During wire localization, the radiologist, or surgeon, will place a wire into or adjacent to the breast lesion under image guidance. This may be performed with either stereotactic, US or MR guidance. This procedure is often performed on the day of operation, as a wire protruding from the skin can be uncomfortable for the patient and is at risk of dislodgement. The surgeon uses the wire as a guide to locate and remove the lesion and the previously placed clip.
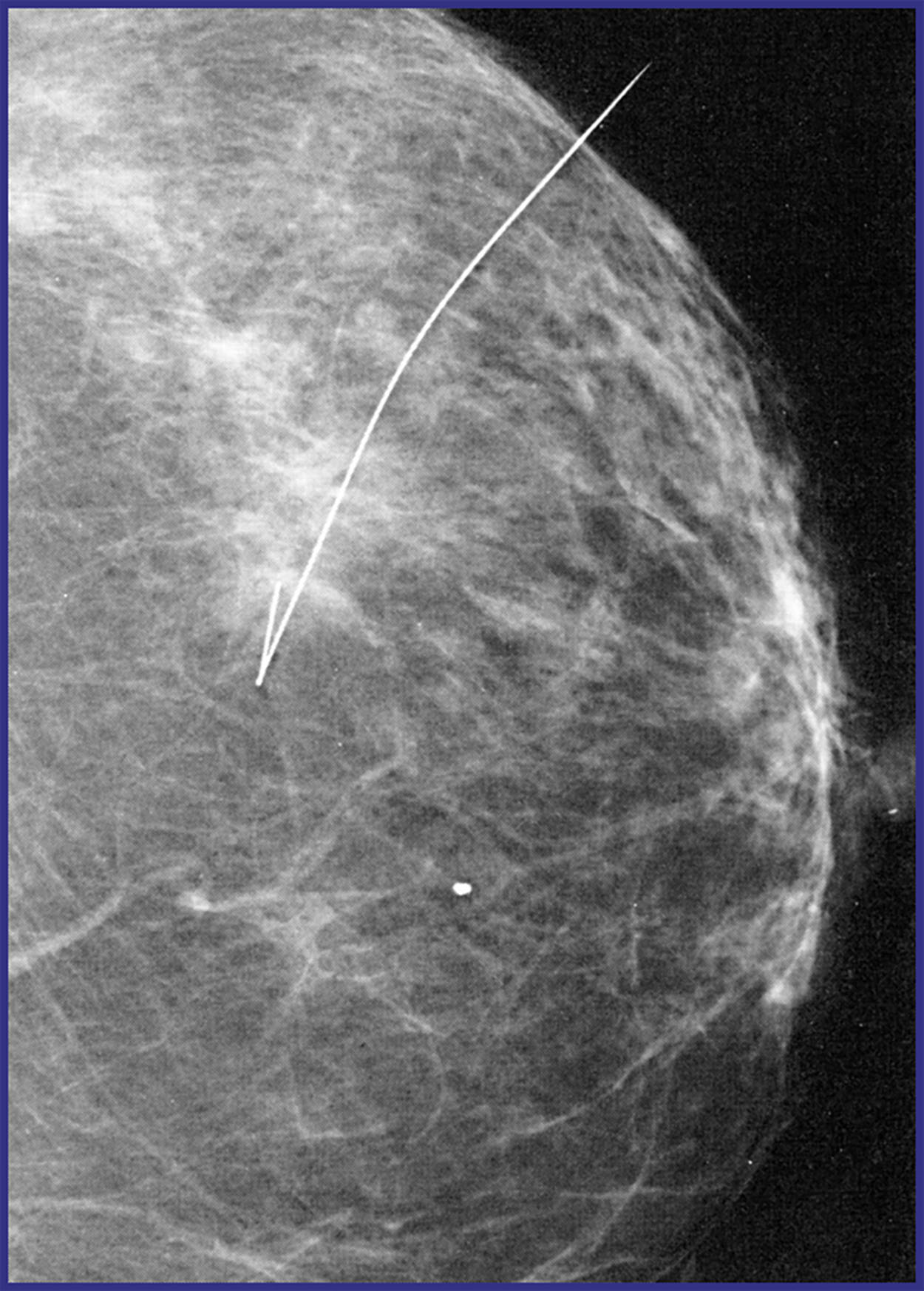
Seed localization utilizing a detectable seed instead of a wire was developed as an alternative to wire localization ( Fig. 7.6 ). Multiple seed localization technologies exist. Radioactive seeds containing iodine-125 were the first on the market and additional technologies followed. In 2014, SAVI SCOUT radar localization from Cianna Medical Inc., and in 2016 Magseed by Endomagnetics Inc., were approved by the US Food and Drug Administration for preoperative breast localization. Most recently in 2017, the LOCalizer by Hologic Inc. was approved by the Food and Drug Administration and uses radiofrequency identification. Axillary lymph nodes may be localized by wire or seed placement in order to perform targeted axillary dissection (TAD). Studies have shown that outcomes favor seed technology and do not significantly differ between the various seed technologies.
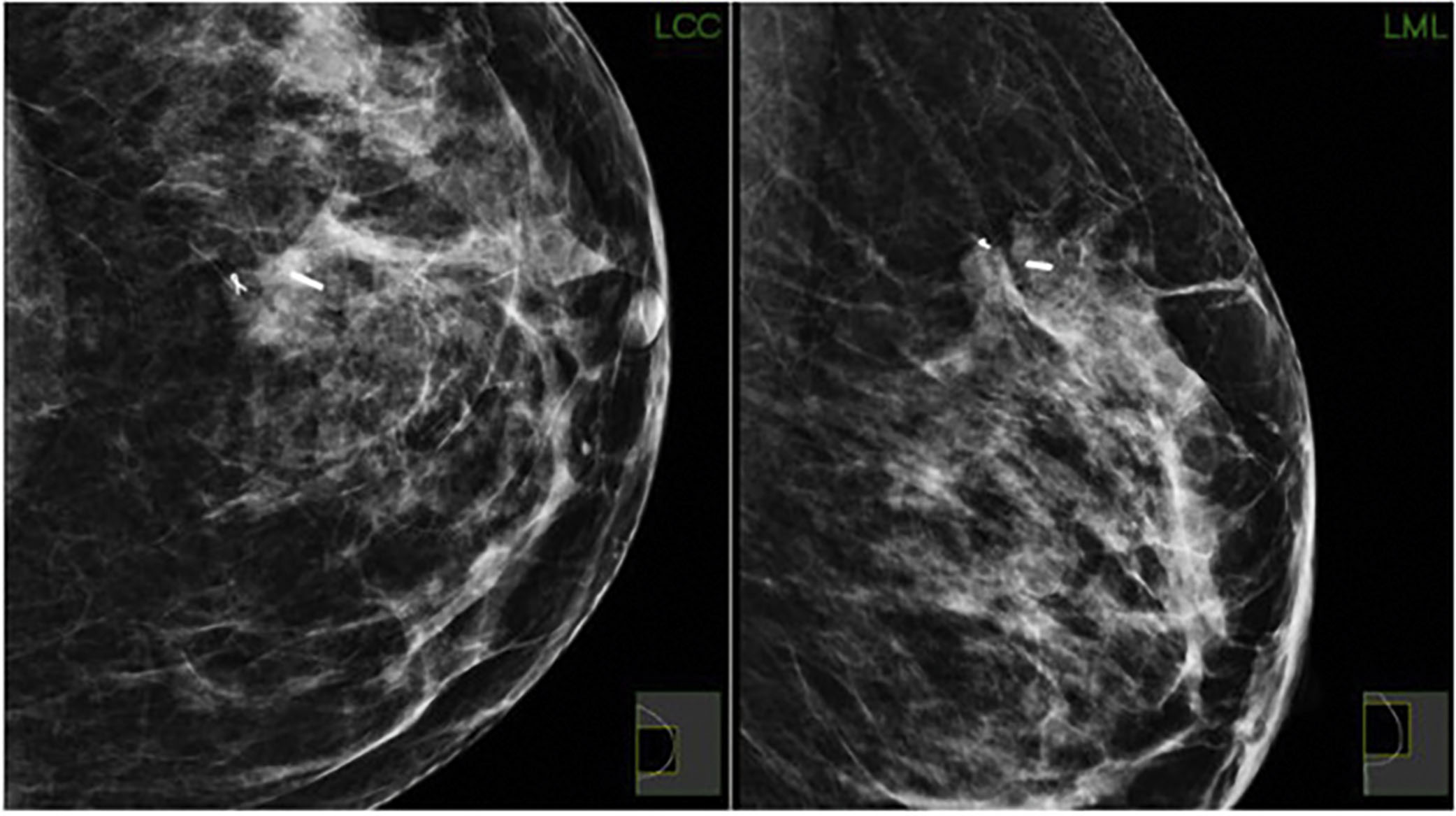
Seed localization is usually performed by the radiologist preoperatively and identified by the surgeon intraoperatively with various probes. Advantages and disadvantages for each type of seed technology exist. The major advantage to seed technology is the ability to place the seed prior to the day of surgery. Additional advantages include improved patient and clinician satisfaction as well as decreased operative times. Disadvantages are specific to the seed technologies themselves. Radioactive seeds are governed by state regulations and facilities require trained personnel and licensing to oversee the program. While radioactive seeds are titanium-encapsulated, the capsule can be transected resulting in the release of radioactive material. Radar seeds can be deactivated by direct contact with electrocautery. While magnetic seeds do not deactivate with electrocautery, metal instruments interfere with detection.
Regardless of device used, review of the preoperative images by the operating team is necessary in order to create a surgical plan. Intraoperative specimen imaging is necessary to ensure seed removal.
Breast Conservation
The decision to proceed with breast conservation depends on patient desire and candidacy for breast conservation. As previously discussed, multiple randomized controlled trials have demonstrated that there is no difference in overall survival when comparing breast conservation to mastectomy. As such, it is vital that patients who are candidates for breast conservation understand that choice of surgery will not impact their survival, but small differences in local recurrence exist.
Absolute contraindications to breast conservation include pregnancy, diffuse malignant or indeterminate calcifications, inflammatory breast cancer, or persistently positive margins. Relative contraindications include multicentric tumors, large tumors relative to the breast volume, or inability to be treated with radiation (i.e., due to genetic mutation, prior radiation, collagen-vascular disease, noncompliance, or absence of a radiation center).
Breast-conserving surgery is also known as segmental mastectomy, partial mastectomy, or lumpectomy. However, segmental or partial mastectomy is the preferred nomenclature, as the lesion of interest is often occult and not a “lump.” As with all procedures, informed consent is obtained prior to the operation. Nonpalpable lesions are localized preoperatively. Preoperative films are reviewed by the surgical team in order to ensure that the correct lesion was localized and to develop an operative plan. Either a periareolar incision or incision over the lesion of interest can be made. The lesion and a small rim of surrounding tissue are removed.
The surgeon may choose to mark the specimen with sutures to denote surgical margins. Alternatively, many centers routinely perform shave margins of the lumpectomy cavity. A single-center randomized controlled trial evaluating shave margins demonstrated a reduced positive margin rate and reduced re-excision rate when cavity shave margins were obtained. Findings were later validated in a multicenter randomized trial. Retrospective evaluation demonstrated the lowest recurrence when both the lumpectomy specimen and cavity shave margins were negative. Interestingly, the highest recurrence rate was seen when the lumpectomy specimen was negative and the shave margins positive. This can be explained by the phenomenon that tumors may not necessarily grow contiguously.
A specimen radiograph is obtained to verify that the lesion, wire or seed, and clip are obtained ( Fig. 7.7 ). The specimen is then sent to pathology for evaluation. The lumpectomy cavity may be closed in multiple layers to occlude the dead space or allow for a seroma to fill the cavity, which can help preserve the normal breast contour. Pathology results are reviewed at the postoperative appointment.
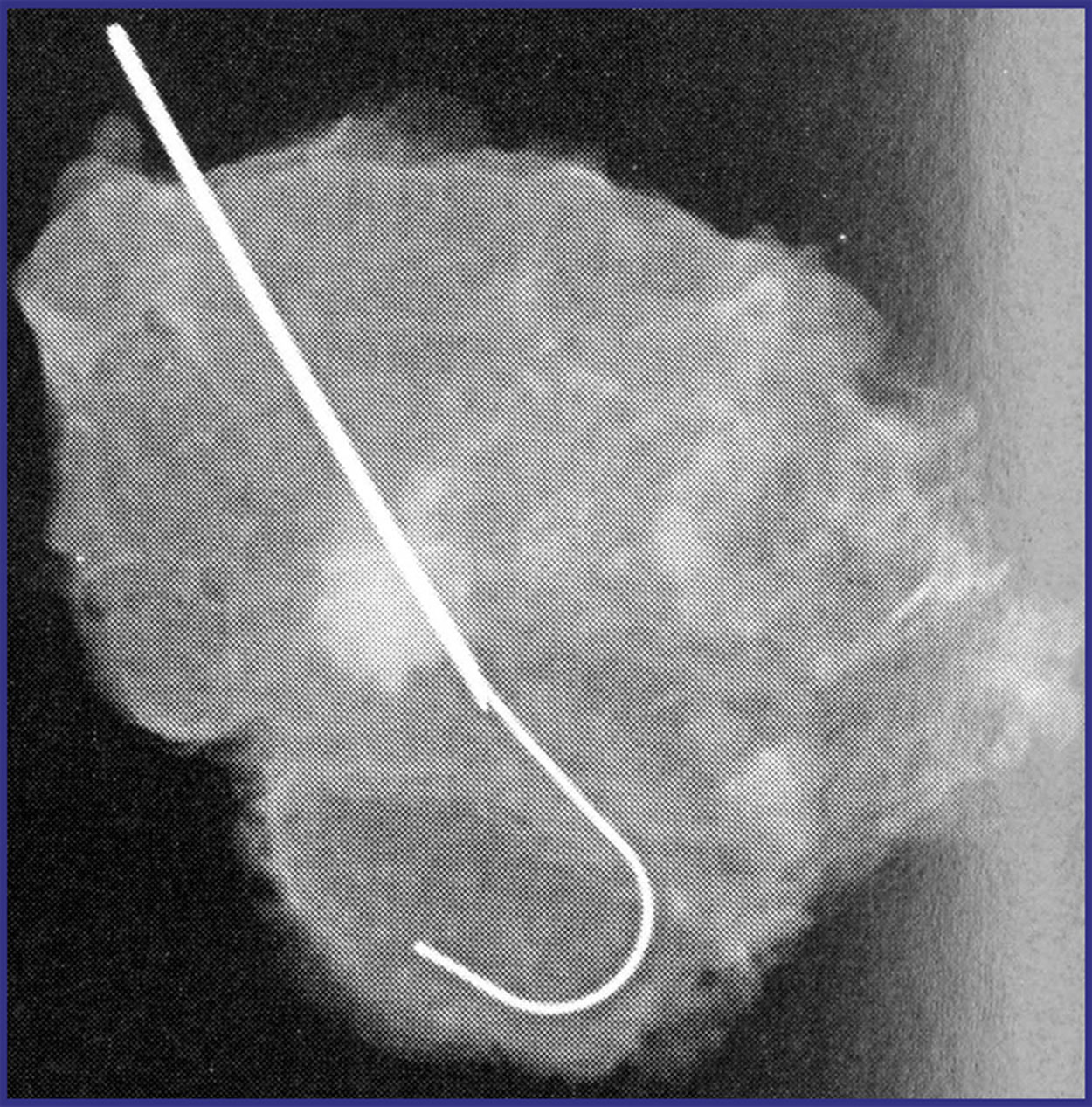
At times, the clip is not obtained in the lumpectomy specimen. Residual clips can contribute to increased patient and surgeon anxiety. When this occurs, it is important to have a discussion with the patient. Excision of the lesion, and not the clip, is the goal of the operation. Postoperative mammography can evaluate for clip dislodgement within the lumpectomy bed or if the clip was lost at the time of surgery. If the lesion of interest was not removed or positive margins remain, re-excision is necessary and the clip can be removed at that time. If the lesion was obtained with negative margins, clip removal is not necessary.
Margins Following Breast-Conserving Surgery
At the postoperative visit, the surgeon reviews the pathology report with the patient, specifically discussing tumor biology and margin status. The Society of Surgical Oncology and American Society for Radiation Oncology have published margin guidelines for breast-conserving surgery for both invasive and in situ disease. For invasive disease, no ink on tumor is considered a negative margin. However for ductal carcinoma in situ (DCIS) a 2-mm margin is recommended. For lesions with both invasive disease and DCIS, invasive margin guidelines should be followed and no ink on tumor is considered sufficient, even when an extensive intraductal component (EIC) is present. However for patients with tumors with EIC, postoperative mammography should be considered to assist in identifying residual calcifications. DCIS with microinvasion should be treated as DCIS with 2-mm margins. When positive margins occur, re-excision is often indicated as positive margins result in at least double the rate of recurrence. However, sometimes re-excision is not possible. These circumstances warrant a discussion with the multidisciplinary team.
Mastectomy
The decision to undergo mastectomy is a highly personal one. A woman may decide to undergo mastectomy for personal reasons or she may not be a candidate for breast conservation. Historically, men with breast cancer were treated with mastectomy; however National Comprehensive Cancer Network guidelines recommend considering breast conservation versus mastectomy in men based on criteria used for women. There are no absolute contraindications to mastectomy aside from inability to tolerate the operation. Women who undergo mastectomy should be offered consultation for reconstruction with a plastic surgeon.
The Women’s Health and Cancer Rights Act was signed into law on October 21, 1998 and mandates that insurance covers all reconstructive operations related to mastectomy, as well as surgery to the contralateral breast for symmetry. Alternatively, women may choose to remain flat following total mastectomy. The “going flat” movement is gaining popularity. For women who desire reconstruction, skin-sparing mastectomy or nipple-sparing techniques may be considered. The nipple-areolar complex is removed in the skin-sparing technique while it is preserved in the nipple-sparing technique. Women are considered candidates for nipple preservation provided the lesion is a sufficient distance away from the nipple. Traditionally this distance was considered 2 cm; however patients may be eligible for nipple-sparing mastectomy even when this distance is less than 2 cm. Women with a prior history of breast surgery, extremely ptotic breasts, or smokers may not be candidates for nipple-sparing mastectomy as they are at higher risk for nipple loss and complications. A delayed nipple flap may increase nipple survival in these women.
Prophylactic contralateral surgery may also be considered for women undergoing mastectomy. However contralateral prophylactic mastectomy does not confer a survival benefit in the general population.
Patients should be counseled preoperatively that a small amount of breast tissue will remain following mastectomy as it is not possible to completely remove all breast tissue. Recurrence after mastectomy, while rare, is still possible and depends on several clinical factors including patient age, tumor biology, and adjuvant therapies. Chest wall recurrence requires multidisciplinary care and management for treatment.
When breast reconstruction is desired, the operation is coordinated with the plastic and breast surgery teams and may be done simultaneously. Ideally the location for incision is decided upon preoperatively with the various teams as well as patient input. For total mastectomy without reconstruction, an elliptical incision encompassing the nipple-areolar complex, and if possible the lesion, is made. A smaller elliptical incision is made either transversely or vertically surrounding the nipple-areolar complex for the skin-sparing technique. For the nipple-sparing technique, a variety of incisions may be chosen to preserve the nipple. When a sentinel node biopsy is to be performed, blue dye or radiocolloid technetium 99 m sulfur colloid is injected prior to making the incision.
Mastectomy may be performed using various techniques. Sharp dissection with or without tumescence or electrocautery may be used. Regardless of the technique, flaps should be created to the level of the clavicle superiorly, latissimus dorsi laterally, inframammary fold inferiorly, and lateral border of the sternum medially. There is no uniform recommendation for flap thickness. Flap thickness depends on the patient’s breast and may differ from one side to the other. The breast is dissected from the pectoralis fascia. Fascia removal is not necessary. If using a nipple-sparing technique, a subareolar biopsy should be performed to assure a tumor-free nipple-areolar complex.
A sentinel node biopsy may be performed through the mastectomy incision or through a separate axillary incision. Some surgeons obtain a frozen section of the sentinel node and if macrometastatic disease is present, completion axillary dissection could be performed. For skin-sparing and nipple-sparing techniques, axillary dissection may require a separate incision. The axillary incision location should take into consideration the location of the breast incision in order to optimize flap perfusion.
The specimen should be oriented. If reconstruction is being performed, the plastic surgery team may begin. If no reconstruction is being performed, then the oncologic surgery team commences with closure. One or two closed suction drains are placed in the mastectomy bed and the tissues are closed in layers. A regional block may be performed at the time of surgery. If pain is adequately controlled, the patient may be discharged from the postoperative suite.
The patient is seen in follow-up where pathology results are reviewed and the drains evaluated for removal.
Sentinel Node Biopsy
The sentinel node technique was developed in the early 1990s for use in melanoma and breast cancer. Sentinel node biopsy is indicated for invasive disease and perhaps in some women with DCIS. There are groups of patients with invasive disease for whom sentinel node biopsy is not routinely performed. The Choosing Wisely guidelines recommend against sentinel node biopsy in women aged 70 years or over with early-stage hormone receptor–positive, HER2-negative invasive breast cancer. However for women 70 years or over who have more advanced disease, sentinel node biopsy may be considered as results may change management decisions. Sentinel node biopsy is not indicated when mastectomy is performed for prophylactic reasons.
The rate of upstage in DCIS has been shown to be as high as 56%, prompting the need to consider sentinel node biopsy for certain patients with DCIS to prevent a return to the operating room should invasive disease be identified. For patients with DCIS, the decision to perform sentinel node biopsy depends on many factors. A mass seen on imaging, higher grade lesions, multicentric disease, and size of lesion have been shown to predict upstaging and are criteria by which to consider sentinel node biopsy for patients with DCIS undergoing breast conservation. When sentinel node biopsy is not performed and final pathology reveals invasive disease, the patient should be consented for return to the operating room for definitive surgery. Sentinel node biopsy should be performed for women with DCIS undergoing mastectomy since it would not be possible after mastectomy.
Sentinel node biopsy can be performed using either blue dye or lymphoscintigraphy, or both. Vital blue dyes include methylene blue or isosulfan blue. Surgeon preference, availability, cost, and complication profile may influence a surgeon’s dye choice. Blue dyes are contraindicated during pregnancy, but the use of lymphoscintigraphy is permissible. Methylene blue is less expensive than isosulfan blue and generally more easily available. While extremely rare, isosulfan blue may result in anaphylaxis. Methylene blue must be diluted in sterile water or D5W (not saline due to its ability to precipitate). Dye or isotope may be injected in the peritumoral, intratumoral, intradermal, subdermal, or subareolar space. Dye should not be injected in the skin. Peritumoral injection should be considered if the lesion is in the upper outer aspect of the breast, as subareolar injection in these situations may result in lack of tracking due to tumor blockage of lymphatics. After injection, the breast is massaged for at least 5 minutes to facilitate lymphatic mapping.
Lymphoscintigraphy is another means by which to map the axilla for sentinel node biopsy. Technitium-99 is injected into the breast preoperatively up to 30 hours before surgery. Intraoperatively a gamma-detecting probe is used to identify the hot node(s).
During sentinel node biopsy, a 2–3-cm transverse incision is made between the pectoralis major and the latissimus muscles 1–2 cm inferior to the axillary hair line. The clavipectoral fascia is incised and the axillary fat is visualized. Arm abduction may facilitate visualization of the blue or hot node. If blue dye is utilized, all blue nodes are removed ( Figs. 7.8 and 7.9 ). If lymphoscintigraphy is utilized, all nodes greater than 10% of the hottest node are removed. Lastly, all palpably abnormal lymph nodes should be removed. Removal of internal mammary nodes is not recommended. Frozen section is generally not recommended for sentinel node biopsy performed during breast-conserving surgery unless the patient was treated with neoadjuvant chemotherapy. Use of frozen section for sentinel node biopsy during mastectomy varies by institution. The axillary incision is closed in multiple layers. Drain placement in the axilla is not necessary when sentinel node biopsy is performed.
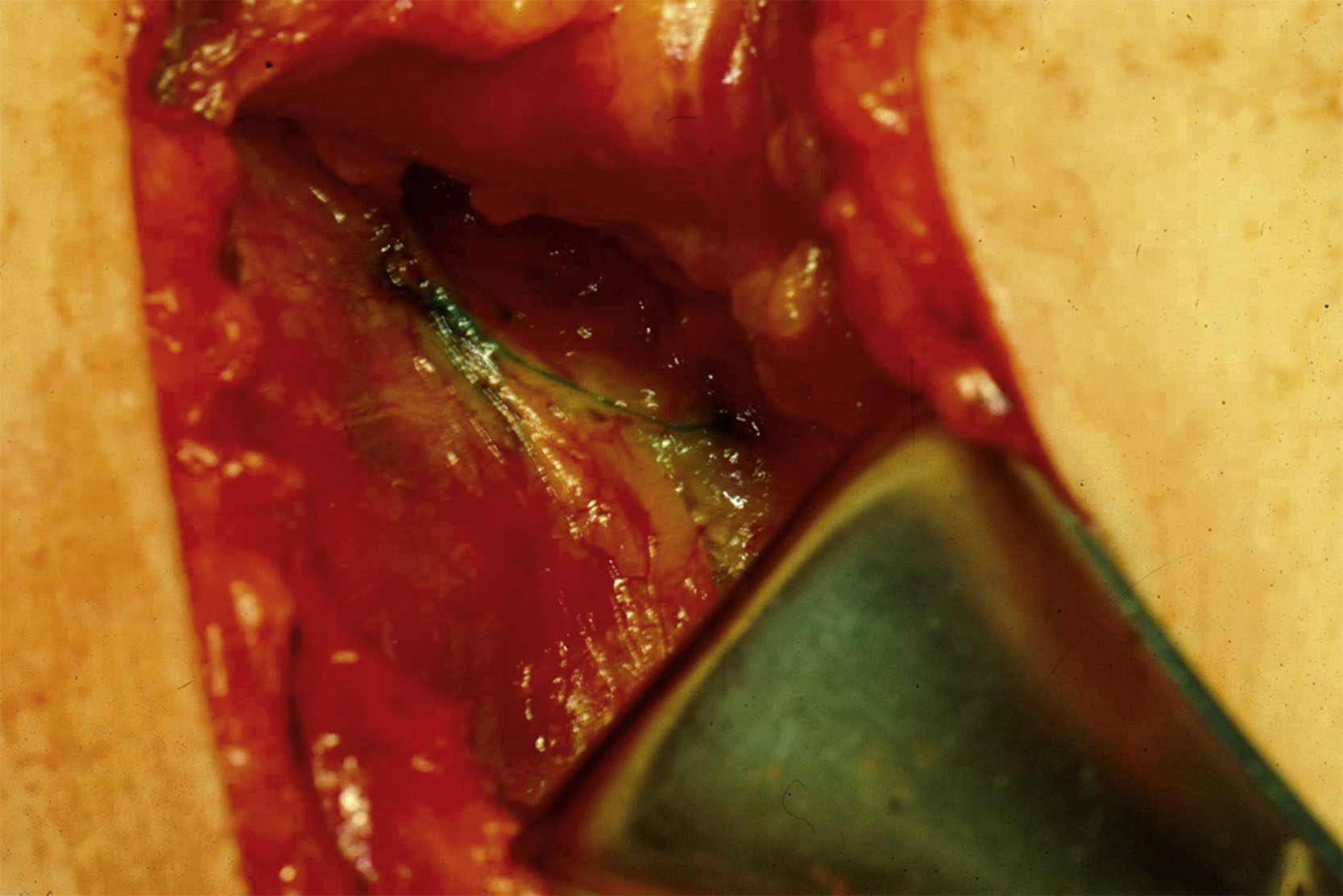
Axillary Dissection
The indications for axillary dissection following breast conservation include three or more positive lymph nodes during breast conservation or any positive lymph node following neoadjuvant chemotherapy. For women undergoing mastectomy who are diagnosed with macrometastasis to the axilla, axillary dissection is indicated. Axillary radiation may be considered as an alternative based on the results from the AMAROS trial. For patients whose lymph nodes contain only isolated tumor cells or micrometastatic disease, axillary dissection is not indicated unless the patient had been treated with neoadjuvant chemotherapy.
Axillary dissection is performed with the patient in the supine position with arms out. Intraoperative movement of the ipsilateral arm may facilitate exposure during the procedure. A transverse incision is made in the axilla and subcutaneous flaps are elevated. The clavipectoral fascia is opened to expose the axillary fat. The axillary contents are removed from the inferior margin of the axillary vein and between the medial margin of the pectoralis minor to the latissimus muscle. There is some disagreement as to the inferior boundary of the axilla. Care must be taken to preserve and ensure functionality of the thoracodorsal and long thoracic nerves during the procedure. The thoracodorsal nerve innervates the latissimus dorsi muscle. The long thoracic nerve innervates the serratus anterior muscle. The long thoracic nerve can be found anywhere up to 1 cm from the chest wall, but it may be inadvertently retracted laterally with the specimen during dissection. The surgeon must be aware of this possibility if the nerve is not identified in its usual location. The axillary contents are removed. A drain is placed within the axilla and the subcutaneous tissues are closed in layers.
Complications
Patients should be counseled on the risk of lymphedema following axillary surgery. This is a complication patients fear most after axillary dissection or sentinel node biopsy. Other complications following breast cancer surgery are uncommon and vary according to the type of operation performed. Infections are the most common postoperative complication related to breast cancer surgery and range from 1% to 20% of cases. As with other types of surgery, age, obesity, and diabetes are risk factors for infection. Preoperative antibiotics may be administered but they should not be administered routinely. Seroma is also a possible complication following breast and axillary surgery, and is more common after mastectomy or axillary dissection. Seroma in the lumpectomy cavity can be advantageous to patients as it can preserve the normal breast contour. Often, small seromas are self-limiting, but if symptomatic, percutaneous aspiration can be attempted. Postoperative hematomas have been reported in 2–10% of cases. With the development of the sentinel node biopsy technique, rates of lymphedema have decreased. Additional complications include chronic pain, breast deformity, flap ischemia, and nerve injury.
Operation After Neoadjuvant Chemotherapy
Neoadjuvant chemotherapy continues to challenge surgical paradigms. As improvements in neoadjuvant chemotherapy increase pathologic complete response, the extent and role of surgery is evolving. Studies are ongoing to evaluate the optimal combination of treatment for these patients.
Sentinel node biopsy is feasible following neoadjuvant chemotherapy. For clinically node-negative patients treated with neoadjuvant chemotherapy, the rate of sentinel node positivity is extremely low. For clinically node-positive women who are treated with neoadjuvant chemotherapy and undergo sentinel node biopsy, it is recommended that the procedure be performed with dual dye, evaluation of at least three lymph nodes, and specimen immunohistochemistry in order to reduce the false-negative rate to appropriate levels. TAD is a procedure during which the sentinel lymph nodes (SLNs) in addition to the biopsy-proven involved lymph node, or clipped node, are removed. Studies have shown that when TAD is performed, the false-negative rate can be reduced to 2–5%. For patients without residual disease, the role of standard axillary dissection and comprehensive radiation in patients with complete pathologic response in the breast and axilla is being explored in the NSABP B51 clinical trial.
When any amount of disease is discovered in the postneoadjuvant patient, even Isolated tumor cells or micrometastases, an axillary dissection is recommended. The findings from AMAROS or ACOSOG Z0011 should not be applied to this cohort of women because residual disease may represent disease that is chemoresistant. The ongoing Alliance for Clinical Trials in Oncology trial 11202 is currently evaluating the role of axillary dissection versus axillary radiotherapy in patients with residual axillary disease.
It has been shown that response to chemotherapy in the breast often correlates with axillary response. The correlation between breast and axillary pathologic complete response appears to be related to pretreatment tumor burden, with near perfect correlation in patients who are clinically node-negative. However, in patients with clinically positive nodes, studies have shown complete nodal response in 45–89.6% with breast pathologic complete response.
For women who are treated with neoadjuvant endocrine therapy, it is generally agreed upon that ACOSOG Z0011 can be applied to this cohort, as residual disease in this group is not felt to represent the same prognosis as is seen with patients treated with neoadjuvant chemotherapy. However there is little data to guide management of patients after neoadjuvant chemotherapy or neoadjuvant hormonal therapy.
Future Directions
Clinical trials exploring de-escalation are evaluating the role of surgery in patients with tumors that respond completely to neoadjuvant chemotherapy. Patients who experience pathologic complete response are referred to as exceptional responders. A study sponsored by MD Anderson Cancer Center is examining the omission of operation after neoadjuvant therapy. The future of breast cancer treatment may evolve to omit operation for patients who are exceptional responders.
Management of the Axilla and Lymphedema
Introduction
Management of the axilla has seen a shift over the years and continues to evolve as our treatments and understanding of its impact on survivorship have changed. There is generally a shift in performing less intervention while still maintaining recurrence and survival rates. The use of axillary surgery and nodal irradiation, which is used for staging and treatment, can result in pain, dysesthesia, weakness, and limited range of motion as well as breast cancer-related lymphedema (BCRL). Breast cancer survivors with BCRL report a long-term decrease in their quality of life as well as chronic pain, depression, and anxiety. They also have higher medical costs and more productive days lost than women without lymphedema. The significant impact on survivorship and frequent requirement for life-long therapy mandates that effective preventive strategies be explored and implemented in our practice regimen.
Definition and Incidence
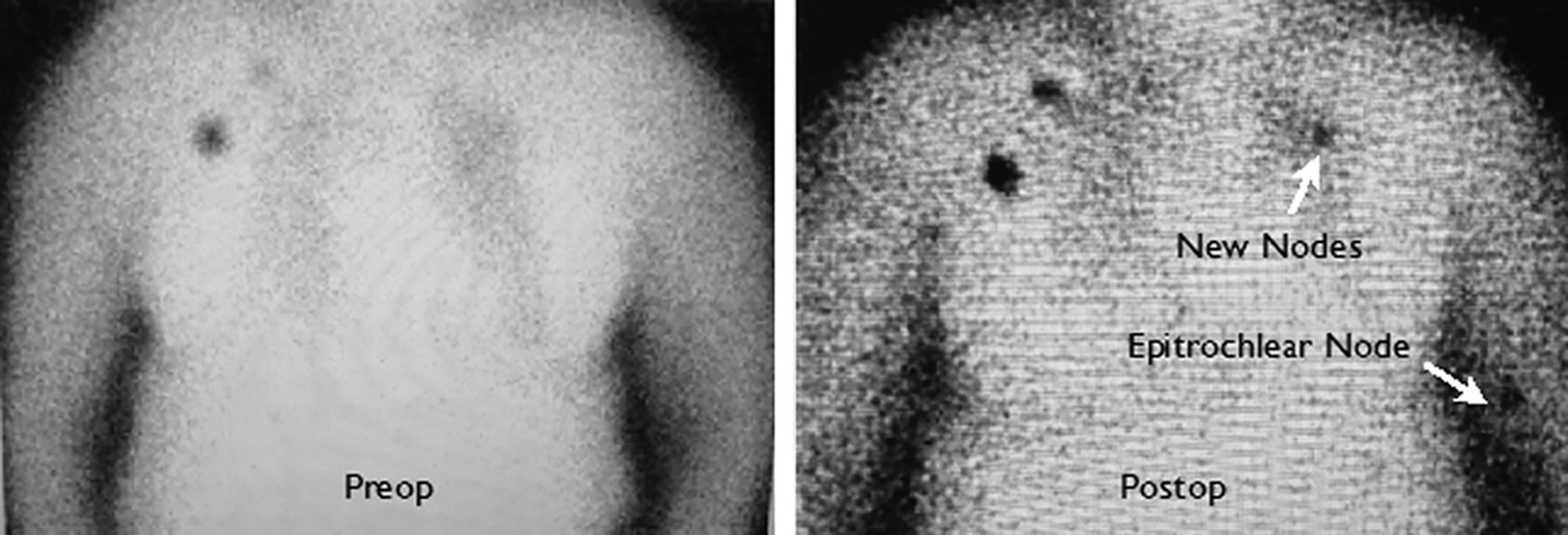
BCRL results from disruption of the lymphatic system that prevents adequate drainage from lymphatic vessels, causing lymph fluid to accumulate in the interstitial space. It has been arbitrarily defined as a 2-cm increase in limb circumference, a 200-mm increase in limb volume, or a 5–10% change in limb volume compared with the unaffected arm. There are four stages of lymphedema (stage 0–3). Stage 0 is subclinical with no visible changes, where patients may report vague heaviness. Stage 1 is mild, soft edema that pits, with no dermal fibrosis, and subsides with limb elevation. Stage 2 is considered moderate, and there is loss of elasticity and evolution of dermal fibrosis with no decrease in swelling with arm elevation. Stage 3 is chronic and irreversible. There is lymphostatic elephantiasis with nonpitting edema and skin changes ( Fig. 7.10 ).
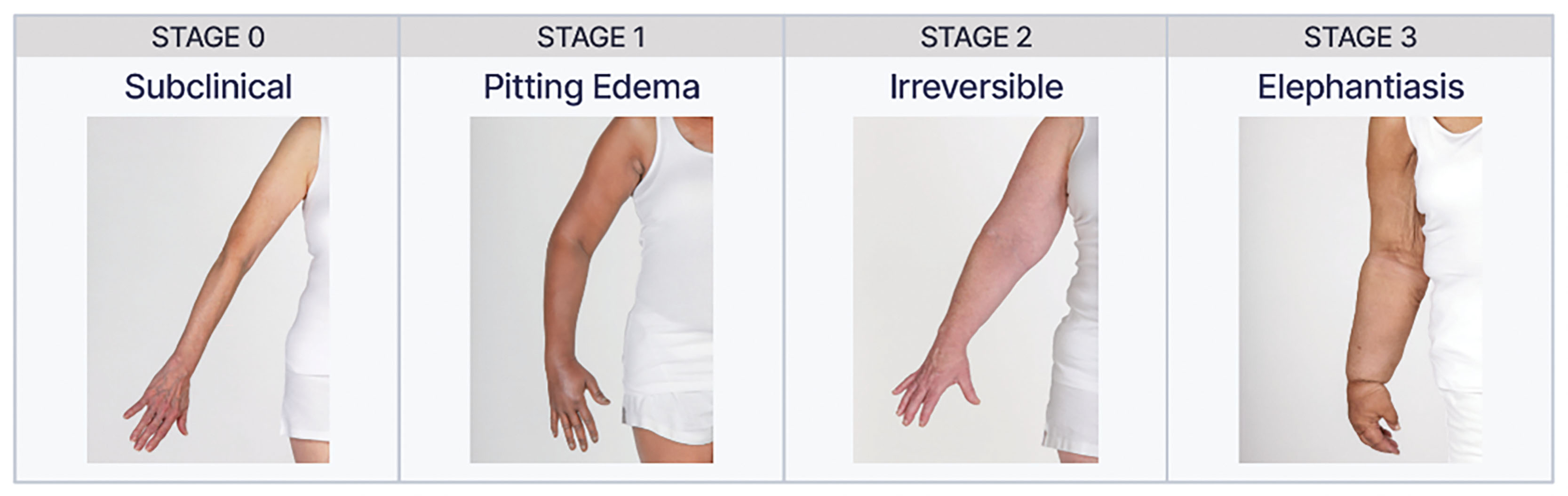
Stages of Lymphedema
BCRL rates range from 0% to 77%, with rates varying based on the type of locoregional and systemic therapies. Sentinel lymph node biopsy (SLNB), while a less invasive dissection, can still have lymphedema rates from 0% to 13%. Lymphedema is the patient’s most feared side effect of axillary surgery, adversely affecting quality of life and job performance. It also dramatically increases healthcare costs.
It is imperative to identify and manage patients at high risk for developing BCRL so they can be monitored and treated before chronic, irreversible BCRL occurs. Multiple studies have found that early detection and treatment of subclinical BCRL can prevent progression to its chronic stage, and eliminate morbidity and the need for more intensive, costly treatments.
Preoperative Assessment and Surveillance
At the earliest stages, diagnosing lymphedema can be challenging. The National Lymphedema Network, the International Society of Lymphology, the National Accreditation Program for Breast Centers, and the National Comprehensive Cancer Network recommend preoperative assessment and ongoing surveillance of the ipsilateral and contralateral arms at regular standardized intervals for those patients at greatest risk of developing BCRL.
There are several methods to detect and monitor lymphedema. There are no head-to-head comparison trials to validate one technique over the other. Therefore objective measures of BCRL include circumferential tape measurement, perometry, water volumetry, and bioimpedance spectroscopy (BIS), all of which present distinct sets of advantages and disadvantages.
Circumferential sequential arm measurements have been traditionally used to identify changes related to lymphedema. Existing guidelines suggest that this is acceptable as a minimum standard provided they are completed with a nonstretch tape measure at multiple points in the arm. Advantages are that it is inexpensive and easily accessible. Disadvantages are that it is time consuming and there is lack of reproducibility, with inconsistent inter- and intrarater reliability leading to inaccurate measurements. Specificity has been reported to be as low as 73%. It also likely does not detect early volume change, but rather a late stage of lymphedema.
Perometry utilizes a frame of infrared light beam-receiver pairs to measure limb outline with subcentimeter definition and thus derive limb volume by the disc model method. The patient places each arm into a large frame that sends infrared light beams inward from different angles. The computer then uses the resulting information to calculate arm volume. It benefits from being quick, accurate, and highly reproducible, but comes at a high upfront cost and large footprint for the equipment. The standard error is found to be smaller and the confidence interval narrower with perometry compared with tape measure.
Water displacement requires that each arm be placed into a large cylinder of water. Any displaced water is measured and compared with the other side. It is accurate and inexpensive; however it does not isolate the site of swelling. In addition it is time consuming, unhygienic, and requires a strict protocol. It has been shown to have an intraclass correlation coefficient of 0.99 for reliability of measurements.
BIS is a simple-to-use, noninvasive tool to assess lymphedema. It determines the quantity of extracellular fluid by measuring tissue resistance to the flow of electric current. A scanning device is used that passes a small, painless electrical current through the limb and measures resistance to the current ( Fig. 7.11 ). Measurements are taken at multiple locations up and down the arm. BIS has been used for both diagnostic and disease-management purposes, as it has high specificity (80–99%). BIS has the ability to detect subclinical BCRL in the clinic with limited time, staff, and space. Limitations of this technique are expense, approximately $5000 for equipment and $12,000 annually for data storage, and potential lack of reimbursement for the test.

The data for BIS has been encouraging. Soran et al. demonstrated that periodic monitoring with BIS reduces the incidence of subclinical BCRL from 36.4% to 4.4%. Similarly Whitworth et al. showed that the use of BIS to facilitate early detection and treatment of BCRL found that 3% of patients had unresolved clinically significant BCRL, which is lower than reported in contemporary studies.
Subjective measures for BCRL include PROs. The usual PROs for BCRL are swelling, pain, heaviness, aching, numbness, stiffness, and impaired arm mobility. Research indicates that PROs should be evaluated at benchmarks during an extended period, 2–6 years after treatment.
Increasingly, data and guidelines support the use of prospective surveillance programs to detect BCRL in its subclinical phase to allow for early conservative treatment to prevent progression to chronic, costly, irreversible phases. Ideally screening programs should use tools that allow for detection of subclinical BCRL in an easy, fast way. The ideal detection tools should be objective, reproducible, and provide a standardized metric that supports treatment decisions. It should also be reimbursable by health plans. For surveillance, an initial preoperative measurement should be obtained followed by regularly scheduled postoperative measurements every 3 months for the first 2 years, then every 6 months for 3–5 years for all patients undergoing axillary surgery.
Reduction of Risk
Risk-Reducing Behaviors
Several risk factors have been identified for development of BCRL. First, it is important to differentiate between lymphedema and lymphatic insufficiency. Patients with lymphatic insufficiency seen by indocyanine green lymphangiography may not have clinically appreciable lymphedema due to compensation by remaining lymphatics. However these patients do have impaired lymphatic function and are therefore at risk for developing clinical lymphedema.
Risk factors are subdivided into patient-specific and treatment-specific risk factors. Patient-specific factors include body mass index (BMI) at the time of diagnosis, subclinical edema, and cellulitis on the side of treatment. The independent treatment-related risk factors for BCRL include lymph node surgery and regional lymph node radiation (RLNR).
BMI has been widely reported to be associated with BCRL. Helyer et al. determined that patients with a BMI greater than 30 kg/m 2 have an odds ratio of 2.93 for developing BCRL compared with patients with a BMI less than 25 kg/m 2 . These findings underscore the importance of patient education and thorough provider-patient discussions during preoperative planning to minimize post-treatment risk of BCRL.
Subclinical edema has been shown to be a risk factor for BCRL. Specht et al. studied the relationship between subclinical swelling and lymphedema. They found that early on, both small- and large-volume increases correlated with increased BCRL. This study, as well as others, highlight the importance of screening for early postoperative arm volume increases. Of note, a patient with subclinical lymphedema likely has lymphatic insufficiency. Regardless of whether the edema is subclinical or clinically appreciable, these patients have BCRL; it is just different points on the spectrum.
Cellulitis is a well-established risk factor for BCRL. A large prospective cohort study by Ferguson et al. emphasized that cellulitis infections significantly increased BCRL risk, with a mean difference in arm volume by about 3%.
Avoidance of ipsilateral venipuncture, blood pressure readings, and air travel has long been considered dogma in the management of BCRL. Many studies, however, have refuted most of these claims and demonstrated that these practices are unlikely to significantly mitigate the development of lymphedema. There is weak evidence to support these behaviors and recently published prospective studies have refuted the use of these practices among at-risk breast cancer survivors. These practices can burden patients with added anxiety and avoidance strategies. Patients adopt these without differentiating between at-risk and affected individuals, with most patients adopting four to five risk-reducing behaviors after axillary surgery.
However while avoidance of these activities may not mitigate against the development of lymphatic insufficiency, they may actually avoid hastening the onset or exacerbation of clinically evident lymphedema. The National Lymphedema Network position paper advises the following for risk reduction: if required to have venipuncture, ask to use a nonlymphedema limb if possible. Use an uninvolved or not-at-risk extremity when taking blood pressure and request a hand blood pressure measurement if possible. Patients can make an informed personal decision regarding use of a compression garment during air travel; regardless it is important to move around, exercise the at-risk body part, and maintain good hydration during air travel. Therefore patients should not be overly cautioned against these things if they are necessary for medical (intravenous, injections) or personal (travel) reasons, but should try to avoid using an affected extremity if not necessary.
De-Escalating Axillary Surgery
Axillary nodal status remains among the most important variables for both prognosis and adjuvant therapy when treating breast cancer. Historically axillary lymph node dissection (ALND) was considered a mainstay in the treatment of breast cancer. However as treatment and staging criteria evolved, we have seen the necessity to perform ALND decrease. With the increased utilization of SLNB and data supporting de-escalation of axillary surgery, ALND has been avoided more commonly. The data from ACOSOG Z0011 and AMAROS have driven this trend. However the rate of lymphedema still exists with SLNB, shown to be as high as 13%.
These findings have motivated the consideration of eliminating unnecessary lymph node analysis in certain circumstances. As part of the Choosing Wisely Campaign, which encourages doctors and patients to question commonly used tests and treatments, the ASBrS recommended against the use of routine ALND in patients undergoing lumpectomy. In addition Choosing Wisely guidelines and the Society of Surgical Oncology recommended against routine use of SLNB in women over 70 years of age with hormone receptor-positive HER2-negative disease. This recommendation is based on data supporting that surgical staging of the axilla is not likely to change systemic therapy recommendations. Hughes et al. showed that the risk of axillary recurrence was low among women who did not undergo axillary surgery. Furthermore there was no difference in breast cancer specific mortality in those who underwent axillary surgery and those without axillary surgery in women over the age of 70 years.
In the majority of patients with DCIS, SLNB may not be necessary. Hung et al. conducted a retrospective study on patients with DCIS who had SLNB and breast-conserving surgery. When compared with those who did not have SLNB, there was no difference in treated recurrence, ipsilateral invasive occurrence, or breast cancer mortality. This is supported in other studies as well.
To aid in this avoidance, the SentiNot study evaluated injection with superparamagnetic iron oxide nanoparticles for patients with DCIS to minimize unnecessary SLNB. Patients who were found to have invasive disease could go on to have SLNB even after mastectomy, as this technique allows SLNB to be performed several weeks after injection. They found that 78.3% of patients with DCIS avoided SLNB.
Axillary Surgery After Neoadjuvant Chemotherapy
Traditionally node-positive patients receive a complete ALND. The American College of Surgeons Oncology Group (ACOSOG) Z1071 was designed to test the hypothesis that SLNB would accurately assess nodal response after NAC. They evaluated over 600 patients who were initially node-positive and received chemotherapy, followed by both SLNB and ALND. They found the false-negative rate (FNR) to be 12.6% by identifying cancer in nodes other than the sentinel node. The use of a dual tracer increased the likelihood of finding a node and lowered the FNR. They also showed that the FNR improved as the number of removed SLNs increased: 31% with one node, 21% with two nodes, and 9.1% when three or more nodes were removed.
The SENTinel NeoAdjuvant (SENTINA) study evaluated the optimal timing of SLNB in patients undergoing NAC. There were four arms to this study, including those who were node-negative before treatment. Arms A and B included patients who were clinically node-negative (cN-) and underwent SLNB before NAC. Arm A patients were SLNB-negative and received no further axillary treatment. Arm B were SLNB-positive and underwent SLNB and ALND after NAC. Arm C and D were clinically node-positive (cN+). They got NAC upfront and nodal status was evaluated afterwards. Arm C patients were those who converted to cN- and got SLNB and ALND. Arm D remained cN+ and went straight to ALND. The investigators concluded that patients should only get one SLNB for staging. The FNR for SLNB was 12.3% in pathologically node-positive (pN+) patients after chemotherapy. Dual tracer versus single brought down the FNR from 16% to 9%. In addition, removal of three or more nodes had a FNR of 7% compared with 24% with only one node removed.
The Sentinel Node Biopsy Following Neoadjuvant Chemotherapy (SN FNAC) study investigated node-positive patients receiving NAC and found the FNR of SLNB was 8.4% overall, with improvement if more nodes were taken.
The GANEA2 study assessed the accuracy and safety of SLNB after NAC. They found the FNR to be 11.3%. For patients with initially involved node, with negative SLNB after NAC, the risk of positive ALND was 3.7%. They concluded that ALND can safely be avoided after NAC in patients who are initially node-negative. In those with initially involved nodes who have no lymphovascular invasion and remaining breast tumor size 5 mm, the risk of positive ALND is 3.7%.
These studies demonstrate low FNR for accuracy in SLNB after NAC. Taking this further, Caudle et al. described a technique to increase the accuracy of SLNB after neoadjuvant therapy by targeting the biopsy-proven lymph node. This technique requires a clip be placed in the sampled node prior to neoadjuvant treatment, and then localization with iodine-125 radioactive seed. This technique decreased the FNR, with the lowest FNR reported at 2%. Interestingly, in 23% of cases the clipped node was not a sentinel node, underscoring the importance of localization in this evaluation. Of note these patients will go on to receive radiation therapy. TAD can also be done using injection of carbon particles, or indocyanine green and use of other localization devices. Simons et al. reported on a prospective registry trial using magnetic seeds to locate clipped nodes and had 100% seed placement and removal. Miller et al. found similar success with magnetic seed for lymph node localization. With reliable SLNB after NAC, aggressive axillary surgery can be avoided.
Axillary Reverse Mapping
Axillary reverse mapping (ARM) is a technique used to distinguish the lymphatic drainage of the arm from that of the breast. It identifies upper-extremity (UE) lymphatics coursing through the axilla to minimize unnecessary disruption of the arm lymphatics while performing lymph node surgery. ARM entails mapping UE lymphatics with blue dye at the time of SLNB mapping in the breast with technetium-99, allowing for differentiation of lymphatics draining the breast (hot) and the UE (blue). Variations in UE lymphatic drainage patterns exist and may coalesce or cross over with those draining the breast ( Fig. 7.12 ).
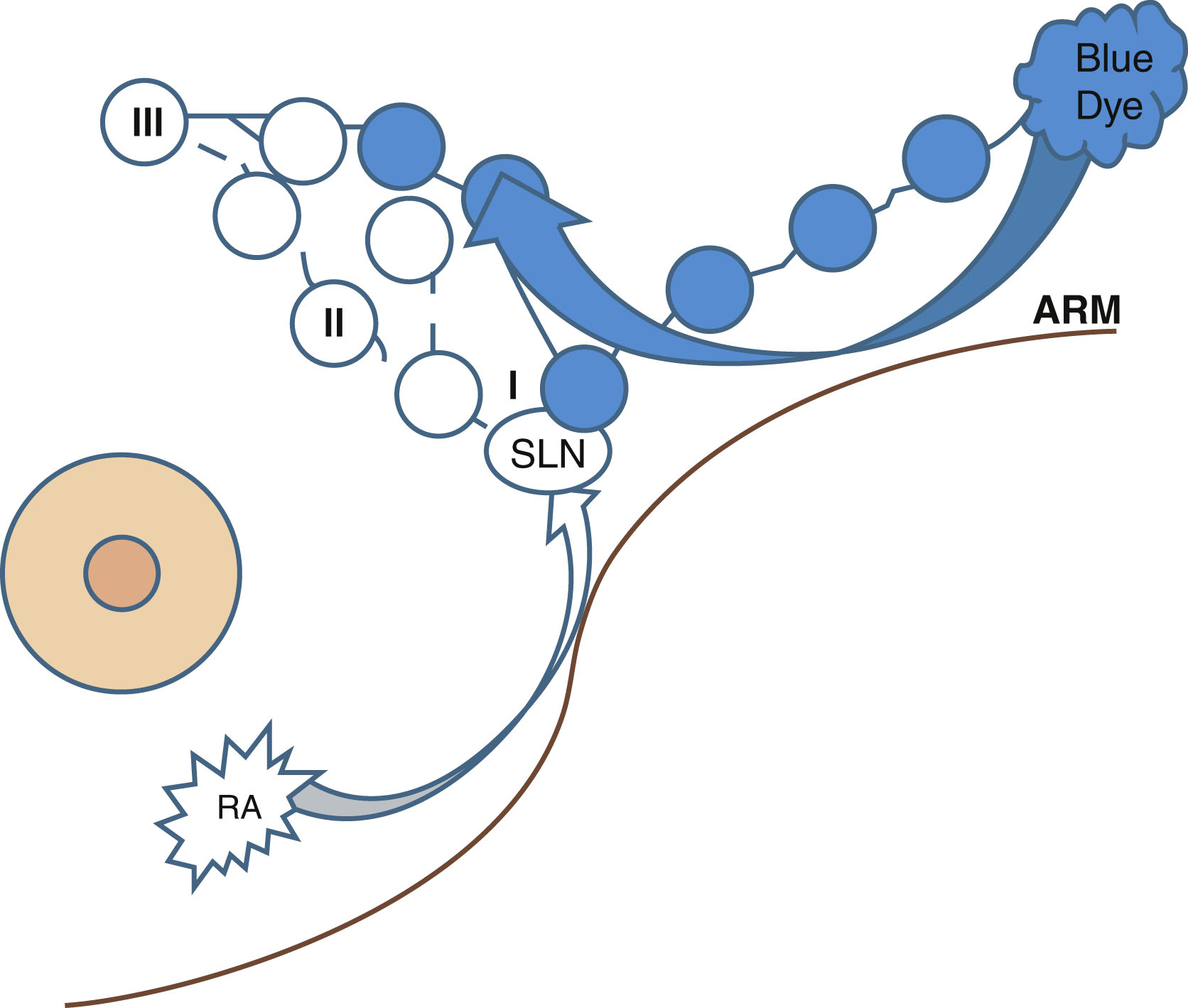
There are three factors to consider with ARM: feasibility, prevention of BCRL, and oncologic safety. A large phase II single-arm trial with 654 patients evaluated the feasibility of ARM. They reported identification of blue UE lymphatics in 29% of SLNB patients and 72% of ALND patients. Boneti et al. identified blue in 39% of those patients having SLNB and in 81% of patients having ALND. Wijaya et al. performed a review and meta-analysis of 29 studies with 4954 patients combined. They found the feasibility to be 37% in SLNB and 82% in ALND. The larger percent of identification in ALND is due to the larger operative field and greater area of dissection, aiding in visibility.
In terms of prevention of BCRL, Tummel et al. prospectively reported on patients in a single-arm study. They found the rate of BCRL after SLNB with ARM was 0.8% and ALND with ARM was 6.5% after 26 months follow-up. In the meta-analysis by Wijaya et al., they found the BCRL rate was 2% in those with SLNB and ARM and 14% with ALND and ARM.
In an analysis of five randomized controlled trials evaluating ALND versus ALND with ARM, all five studies favor ALND plus ARM for lower BCRL rates.
In a small fraction of patients, the ARM (blue) node will also be the SLN. This can call into question the oncologic safety. Tummel et al. found that crossover SLN (hot) and blue was seen in 3.8% of SLNB procedures. Crossover happened in 5.6% of those undergoing ALND. In the subset of patients in which an identified blue lymphatic was transected, there was an overall lymphedema rate of 18.7% when not reanastomosed compared with 0% when reanastomosed at 14 months of follow-up. Of note, the axillary recurrence rate was 0.2% in those with SLNB and 1.4% in those with ALND.
Future work includes the Alliance trial A221702, a randomized prospective phase III trial that studies how well ARM works in preventing lymphedema in patients with breast cancer undergoing ALND. Patients will be randomized to ARM versus no ARM. This randomized trial will help establish change in practice as needed, based on the evidence found.
Lymphatic Microsurgical Preventive Healing Approach
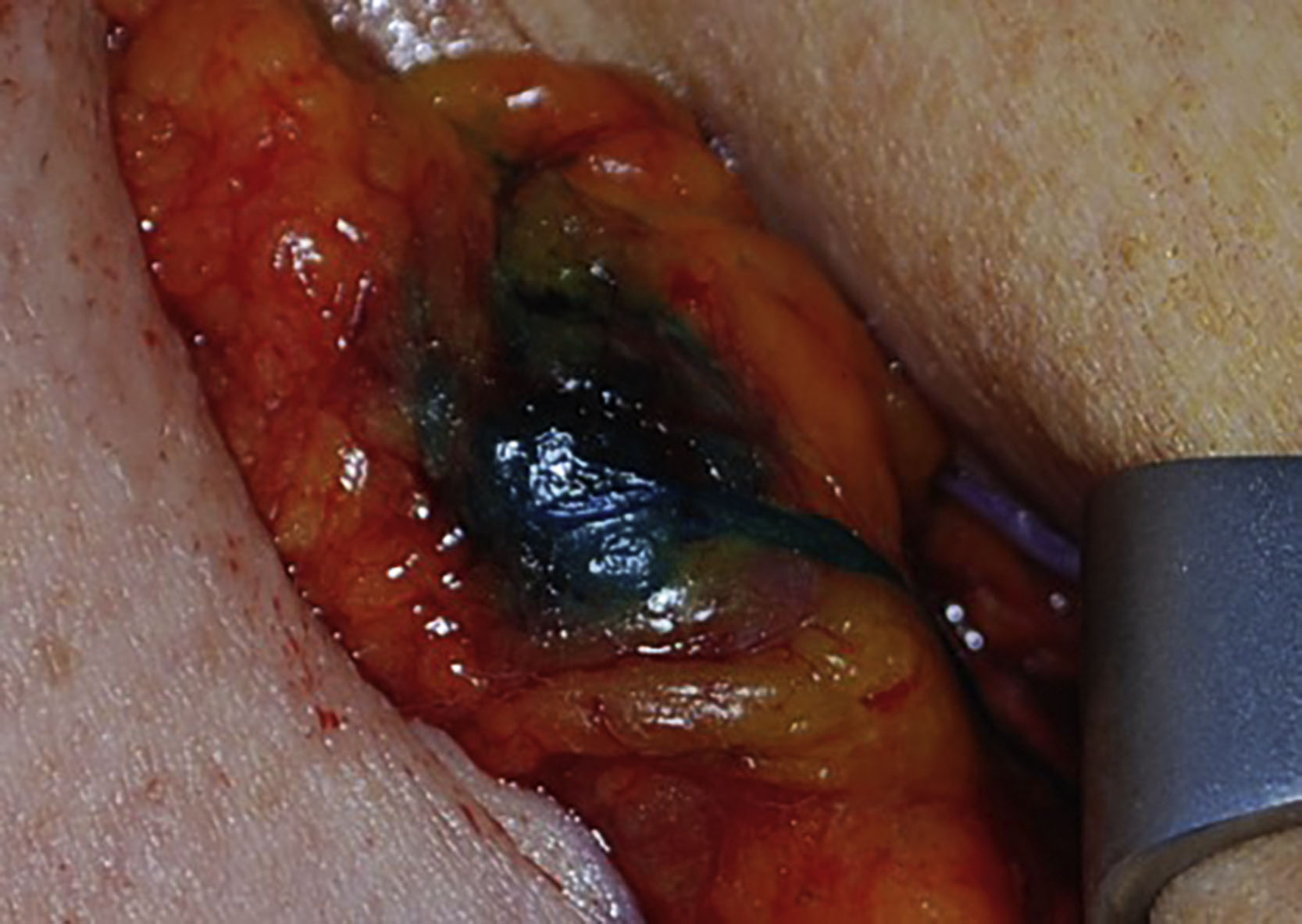
A logical progression from ARM is the lymphatic microsurgical preventive healing approach (LyMPHA) procedure. LyMPHA provides a preventive lymphavenous anastomosis. In those patients where the malignant node is found to be draining the arm, a lymphovascular anastomosis can prevent secondary lymphedema ( Fig. 7.13 ). Boccardo et al. described the technique in the Annals of Surgical Oncology : in a randomized study in 2011, Boccardo et al. compared LyMPHA in 46 patients undergoing complete ALND. At 6 months of follow-up, he showed that one (4.34%) patient with LyMPHA developed lymphedema versus seven (30.43%) control patients. Long-term follow-up at 4 years showed no sign of lymphedema in 71 of 74 (96%) of patients who underwent LyMPHA. Feldman et al. found the lymphedema rate was three of 24 (12.5%) in successfully completed and four of eight (50%) in unsuccessfully treated patients. They found LyMPHA to be feasible, safe, and effective for the primary prevention of BCRL.
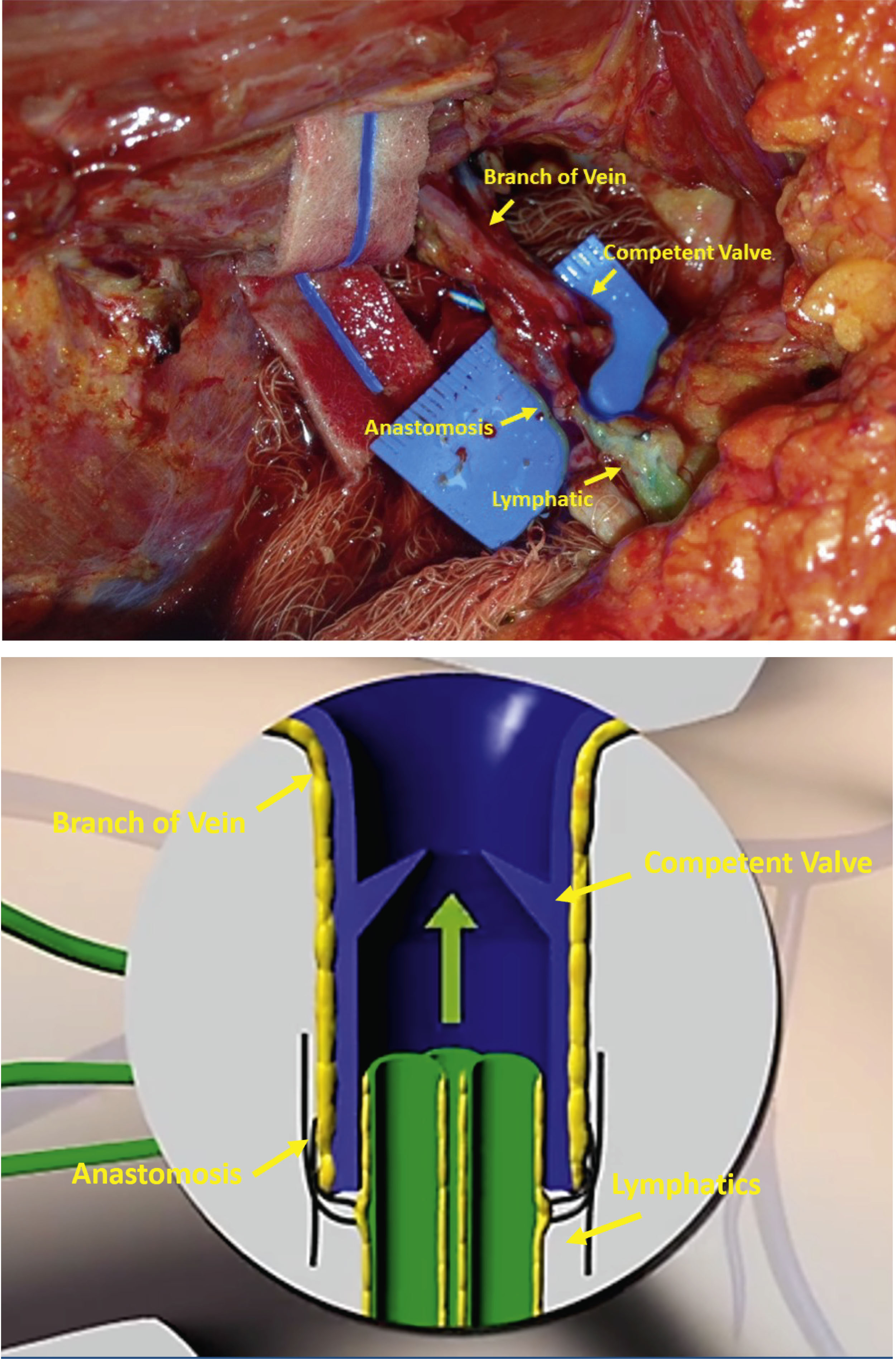
Ozmen et al. evaluated the simplified lymphatic microsurgical preventing healing approach (S-LyMPHA). S-LyMPHA is a slightly modified and simplified version of LyMPHA. It is performed by the operating surgeon performing the ALND. During the ALND, transected blue lymphatic channels are identified and at the completion of the dissection, those channels are carefully dissected and invaginated using a sleeve technique into the cut end of a neighboring vein with two 7–0 nonabsorbable stitches. The microscope is not used in any part of this surgery. They evaluated 380 patients who underwent ALND and found a significantly lower rate of lymphedema in both univariate and multivariate analysis (3% vs. 19%.)
Johnson et al. recently described the benefits of LyMPHA in those patients undergoing ALND and nodal radiation. They performed a literature review and included 19 studies. The pooled cumulative incidence of lymphedema was 33.4% in those undergoing ALND and RLNR versus 10.3% in those undergoing ALND, RLNR, and LyMPHA (P = .004).
ARM and LyMPHA help prevent BCRL by identifying nodes draining the arm, as well as establishing a reconnection for the disrupted lymphatics. Further randomized multicenter trials would need to be conducted to establish more long-term data.
Less Radiation to Prevent Lymphedema
Partial Breast Irradiation
For patients who undergo lumpectomy, radiation therapy is usually recommended. Traditionally whole-breast radiation is recommended; however there is data to show that in patients with early-stage, favorable breast cancer, partial breast irradiation (PBI) is safe and effective. PBI can be delivered as external beam radiation therapy (EBRT), as targeted intraoperative radiation (TARGIT-IORT), balloon catheter, or interstitial catheter brachytherapy ( Fig. 7.14 ). Intraoperative radiation can be given as low-energy x-rays or as intraoperative electron radiotherapy. There have been two large, prospective randomized trials comparing whole-breast radiation to both forms of IORT, electron and 50 kV photons, showing low local recurrence rates and excellent overall survival outcomes.
There are now long-term survival and local control outcomes from the TARGIT-A trial. Vaidya et al. randomized patients to a one-time dose of external beam of whole-breast radiotherapy or TARGIT-IORT immediately after lumpectomy in the operating room. With a median follow-up of 8.6 years and maximum follow-up of 18.90 years, no statistical difference was found for local recurrence-free survival, mastectomy-free survival, distant disease-free survival, overall survival, and breast cancer mortality. Of note, mortality from other causes was significantly lower in the TARGIT-IORT group. This group has previously published on radiation-related quality-of-life parameters after TARGIT-IORT. They found that patients receiving IORT alone reported less general pain and arm symptoms as well as better role functioning than those in the EBRT group. Most common issues were pain in the arm or shoulder and difficulty moving or raising the arm sideways. The frequencies of moderate or severe breast and arm symptoms reported by patients in each group for swelling in the arm versus hand were 8% versus 4% with TARGIT-IORT compared with 9% versus 7% with EBRT. The TARGIT-A trial has not yet reported specifically on BCRL. We hypothesize that since RLNR is avoided with TARGIT-IORT, the BCRL rate will likely be lower compared with EBRT. A review of the radiation fields used in the ACOSOG Z0011 trial found that in approximately half of patients in both study arms, the radiation fields were within 2 cm of the humeral head, possibly causing substantial incidental axillary irradiation. Lymphedema rates from TARGIT-A would be valuable data to be able to identify ways to prevent lymphedema with more directed radiotherapy.
The ELIOT study had a similar design to TARGIT-A, but used electron technology. It randomized 1305 women between whole-breast radiation and single-dose intraoperative electron radiotherapy. Median follow-up was 5.8 years and there was no difference in overall survival or breast cancer recurrence.
Obi et al. reported outcomes and acute toxicities from a single institution with intraoperative radiation. They analyzed 201 patients with a median follow-up of 23 months and found the rate of arm lymphedema was 0.5% ( n = 1) in their group.
Warren et al. conducted a prospective cohort study of 1476 breast cancer patients using volume measurements with a perometer to assess the impact of various radiation techniques on rates of BCRL. At a median follow-up of 25.4 months, the 2-year cumulative incidence of BCRL was 6.8%. Cumulative incidence by radiation therapy type was as follows: 3.0% no radiation; 3.1% breast or chest wall alone; 21.9% supraclavicular; and 21.1% with supraclavicular and posterior axillary boost. Of interest they treated 6% of patients with PBI. In a univariate analysis for factors with risk of lymphedema, those who received PBI only had a hazard ratio of 0.38; however this was not statistically significant given the low number of patients. This highlights the potential benefit of PBI in avoiding RLNR and decreasing the risk of BCRL from radiation. This has been suggested in other studies as well.
Omit Radiation in the Elderly and Neoadjuvant Responders
CALGB 9343 randomized women older than 70 years with T1N0M0 ER-positive breast cancer who received lumpectomy without any axillary surgery at all to either tamoxifen plus radiation therapy or tamoxifen alone. While the tamoxifen plus radiation therapy group experienced greater locoregional control—98% versus 90% in the tamoxifen only group—there were no differences in cancer-specific survival, overall survival, or breast-preservation rates. They concluded that women over the age of 70 years with favorable, small, estrogen-responsive tumors could avoid radiation if they commit to taking endocrine therapy.
Future work in this realm includes the NSABP B-51/RTOG 1304 trial, a randomized phase III trial evaluating the role of radiation therapy for patients who have positive lymph nodes prior to NAC who convert to pathologically negative nodes after NAC. We look forward to the results of this trial to help us to continue to improve our goal of reducing rates of BCRL.
Post Lymphedema Treatment
Nonsurgical Management of Arm Lymphedema
For patients in which lymphedema has been established, there are both nonoperative and operative management options. The mainstay of nonoperative treatment is complex decongestive therapy. There are two phases consisting of reduction and maintenance. The reduction phase includes manual lymph drainage, sequential gradient pump, exercises, low-stretch bandages, and skin care. The maintenance phase consists of compression garment, exercises, and skin care.
There has not been one strategy identified as the most beneficial. A randomized controlled trial tested complex decongestive therapy over standard compressive therapy. There was no significant difference in the percent volume reduction of the arm at 6 weeks and 52 weeks. In addition, reports of quality of life did not differ. Compression garments have been shown to prevent progression in subclinical BCRL as well as reduce arm volume. Manual lymphatic drainage is important for volume reduction. It has been shown to be beneficial to patients with mild-to-moderate BCRL in conjunction with compression bandaging.
Physical therapy by lymphedema-trained therapists has been shown to be more beneficial than just patient education and physical therapy alone. Aerobic and resistance exercise does not incite or exacerbate BCRL. The data suggests that monitored management with trained physical therapists is better than self-directed treatment.
Two randomized controlled trials looked at the role of early detection and intervention. A study from Madrid enrolled 120 patients without BCRL who had ALND. They were randomized to a program of manual lymphatic drainage, massage, and exercise or BCRL education alone. At 1-year follow-up, 25% of patients randomized to education alone developed BCRL compared with 7% in the other group. Another study randomized 65 women without BCRL who had ALND to prospective monitoring and treatment with physiotherapy or surveillance alone. They found at 2 years the incidence of BCRL was 11% in the early intervention group compared with 30% in those with surveillance alone. This data supports the recommendation that early detection and monitored treatment are beneficial at preventing progression.
Surgical Treatment of Arm Lymphedema
Surgical management is another option, particularly for patients who do not respond to noninvasive treatment. There are two main surgical strategies: ablative and physiologic procedures.
Ablative procedures reduce limb volume by surgically removing edematous tissue. Liposuction or suction-assisted protein lipectomy are used as volume-reduction treatments because they are less invasive than older debulking procedures and do not require skin grafting. Liposuction/suction-assisted protein lipectomy has been shown to have significant volume reductions. However, compression garments must be worn continuously to maintain the decreased volume.
Physiologic procedures treat the etiology of BCRL by re-establishing and/or redirecting axillary lymphatic flow. Reapproximation or rerouting of lymphatic drainage pathways can be achieved by establishing unobstructed connections with distal healthy tissue or proximal venous tissues.
Procedures utilizing distal tissues generally involve lymphatic grafts or vascularized flaps containing lymphatic soft tissue. The lymphaticolymphatic bypass procedure involves an anastomosis between healthy lymphatic tissue of the lower extremity to the affected arm’s axillary lymphatics and supraclavicular lymphatics. This has been shown to produce long-term patency and reduce UE volume.
Another major surgical treatment involves vascularized lymph node transfer. A lymph node flap is harvested with its vascular supply from a donor site and introduced into the affected extremity. Blood supply is achieved through an anastomosis from the lymph node flap’s blood vessels to the native axillary blood vessels. There has been reduction in limb volume following this procedure, circumference differentiation of 7.3%, and reduction rate of 40.4% at a mean follow-up of 39.1 months. Furthermore by mapping the lymphatic drainage of the donor site, selective removal of lymph nodes can be achieved to decrease the risk of lymphedema in the donor site ( Fig. 7.15 ).