Grade
Clinical features
0
No signs and symptoms
1
Painless ulcers, edema, or mild soreness
2
Pain and ulcers, but can maintain ability to eat
3
Ulcers, unable to eat due to mucositis
4
Ulcers, need for parenteral or enteral support
Grade | Clinical examination | Functional/symptomatic |
|---|---|---|
1 | Erythema of the mucosa | Minimal symptoms, normal diet |
2 | Patchy ulceration or pseudomembranes | Symptomatic but can eat and swallow modified diet |
3 | Confluent ulcerations or pseudomembranes, bleeding with minor trauma | Symptomatic and unabIe to adequately aliment or hydrate orally |
4 | Tissue necrosis, significant spontaneous bleeding, life-threatening consequences | Symptoms associated with life-threatening consequences |
5 | Death | Death |
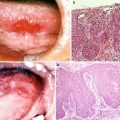
A sensitive, objective, and reproducible scoring system that can be widely applied is greatly needed (Fig. 14.1). Risk factors include the elderly, existing periodontal disease, poor diet, alcohol use, tobacco use, certain medications, oxygen therapy, and changes in breathing [7].
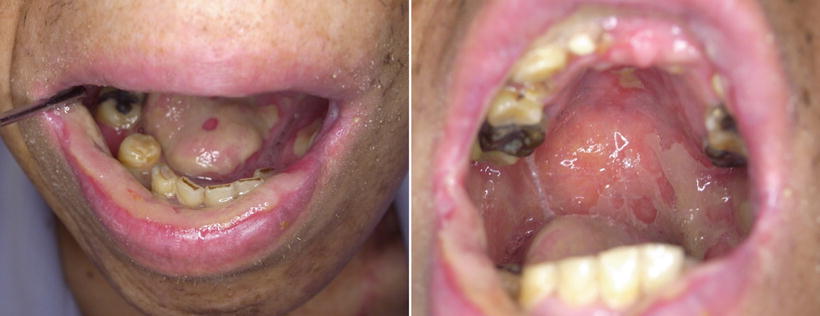

Fig. 14.1
Acute mucositis of the whole mucosa of the oral cavity and the oropharynx following postoperative chemoradiotherapy; 4 weeks of irradiation (approximately 40 Gy) combined with 5-Fu and CDDP for the tongue carcinoma. The mucositis scale of WHO and NCI is grade 3. The patient became dependent on feeding formulas through gastric tubes
There is no universally accepted standard therapy for the prophylaxis or treatment of cancer therapy-induced oral mucositis. Numerous local and systemic approaches to the treatment of mucositis are available for the management of less severe grades of mucositis and consist of palliative relief of pain, an adequate nutrition level, and the monitoring and treatment of localized infections [8]. Liquid forms of systemic analgesics and antibiotics may be easier for the patient to swallow.
Topical coating agents can be most effective in promoting mucosal wound healing. Initially, the tissue must be cleansed of mucoid debris before the application of the agents. An oral liquid suspension can be used if the mouth is dry. All prostheses are to be removed during the oral-mucosal treatment. Topical liquid anesthetics such as 2 % viscous lidocaine may provide temporary analgesia. The suppressive effect on the gag-cough reflex leading to possible aspiration must be explained to the patient before using such topical anesthetic solutions. Prednisone, 40–80 mg per day prescribed for 1 week or less, may help to resolve some of the inflammation [9]. Varied rinses of sodium chloride and sodium bicarbonate may allow for tissue cleansing, moistening, and lubricating. These rinses, along with proper oral hygiene and hydration, are mainstays of prevention and treatment. Any oral medications that contain alcohol, thymol, eugenol, or phenol, which are part of most commercial mouthwashes, should be avoided because they can irritate and desiccate inflamed, compromised xerostomic tissues [10, 11].
14.1.2 Xerostomia
Xerostomia has been reported in association with radiotherapy (RT), chemotherapy, and some immunotherapies involving the major salivary glands. In radiotherapy, clinically, xerostomia has been reported with as little as two or three doses of 2 Gy, although many changes occurring with less than 60 Gy are reversible. However, doses greater than 30 Gy can cause permanent xerostomia [12]. The mean radiation doses that have been associated with permanent impairment of parotid gland saliva secretion are approximately 24 Gy for unstimulated saliva and 26 Gy for stimulated saliva [13]. Scoring systems that grade the severity of acute and late radiation-induced salivary hypofunction have been published by several organizations, including the NCI (Table 14.3) [14].
Table 14.3
Radiation therapy oncology group (RTOG)—European Organization for Research and Treatment of Cancer (EORTC) scoring criteria for radiation-induced salivary gland morbidity [6]
Grade | Acute morbidity | Late morbidity |
|---|---|---|
0 | No change over baseline | None |
1 | Mild dryness, slightly thickened saliva, and slightly altered or metallic taste | Slight dryness of mouth with good response to stimulation |
2 | Moderate to complete dryness, thick sticky saliva, and markedly altered taste | Moderate dryness of mouth with poor response to stimulation |
3 | Not defined for acute xerostomia | Complete dryness of mouth with no response to stimulation |
4 | Acute salivary gland necrosis | Fibrosis |
Xerostomia (dry mouth) changes the ability of the mouth to neutralize acid, clean the teeth and gums, and protect the mouth from infection. It is associated with an increased risk of radiation caries, which results from an increased number of caries-forming bacteria in the oral cavity, low salivary PH with loss of buffering capacity, decreased mechanical flushing, and decreased production of salivary proteins, immunoglobulins (i.e., IgA and IgG), lysozymes, and peroxidases [15]. Accumulation of plaque and other debris on teeth and periodontal tissues resulting from xerostomia may develop to a greatest risk of osteoradionecrosis [16]. Symptoms include dryness, a sore or burning feeling (especially on the tongue), cracked lips, cuts or cracks at the corners of the mouth, and changes in the surface of the tongue. As saliva is needed for taste, swallowing, and speech, xerostomia is associated with the development of dysgeusia or ageusia [11, 17]. An extremely dry mouth will also impair proper speaking, wearing dentures, mastication, and the swallowing of foods.
To minimize the severity of xerostomia and oral complications, it is important to begin aggressive oral care before RT. Appropriate nutritional intake, effective oral hygiene, and early detection of oral lesions are important pretreatment practices. Evaluation in several weeks advance of therapy is essential to determine oral health status, perform necessary dental and oral interventions, and allow time for healing from any invasive procedures that are required. In particular, attention should be given to mucosal lesions, dental caries and endodontic disease, periodontal disease, ill-fitting dentures, orthodontic appliances, temporomandibular dysfunction, and salivary abnormalities. A stringent oral hygiene program is critical and should be continued before, during, and after therapy [11].
Frequent sips of water and water rinses are most commonly used and are essential for partial control of radiation-induced xerostomia. Patients are instructed to carry with them plastic bottles of water for constant use. Sugarless chewing gum and tart candy may be helpful. In some patients, pilocarpine hydrochloride solution (1 mg/mL) or Salagen tablets (5 mg) have been effective in stimulating saliva production [18]. Five milligrams, three or four times daily, has been an optimal dosage for most patients. The most common side effects include sweating, a “flushed” feeling, and stomach discomfort, but these usually occur only at higher dosages [19].
14.1.3 Osteoradionecrosis
Osteoradionecrosis (ORN), which is defined as necrosis of the bone in areas that have received radiotherapy, is one of the most serious complications of the postradiotherapy patient. Bone cells and vascularity may be irreversibly injured, and when ORN is progressive, it can lead to intolerable pain and interference with function or fracture. The preventive and therapeutic use of antibiotics and hyperbaric oxygen (HBO) can be effective, but reproducible beneficial results remain uncertain [20]. The incidence of ORN varies from 4 % to 22 % depending on the reporting institution, aggressiveness of radiotherapy, and follow-up time [21–23].
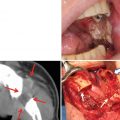
The risk of developing spontaneous ORN is somewhat unpredictable but is related to the dose of radiation. Patients who have received doses of radiation in excess of 60 Gy have the highest risk of this pathological entity. Irradiated areas of bone supplied by damaged vessels with lack of oxygen and nutrients become ischemic and ultimately necrotic. The mandible is affected more frequently because it has less of a blood supply than the maxilla. Obviously, acute or chronic irritation or trauma to avascular mucosa and bone increases the risk of tissue breakdown and ORN. The risk seems to be increased in dentulous patients, even more so if the teeth within the treatment field are removed after therapy [21]. Spontaneous bone exposure usually occurs more than 1 year after radiation is completed. Any oral surgery procedure increases the risk of the development of spontaneous ORN, even if it is performed years after the last radiotherapy treatment (Fig. 14.2). The risk of ORN will be present for the remainder of the patient’s life. Unfortunately, the passage of time does little to reverse the damage and the subsequent risk of ORN [24].
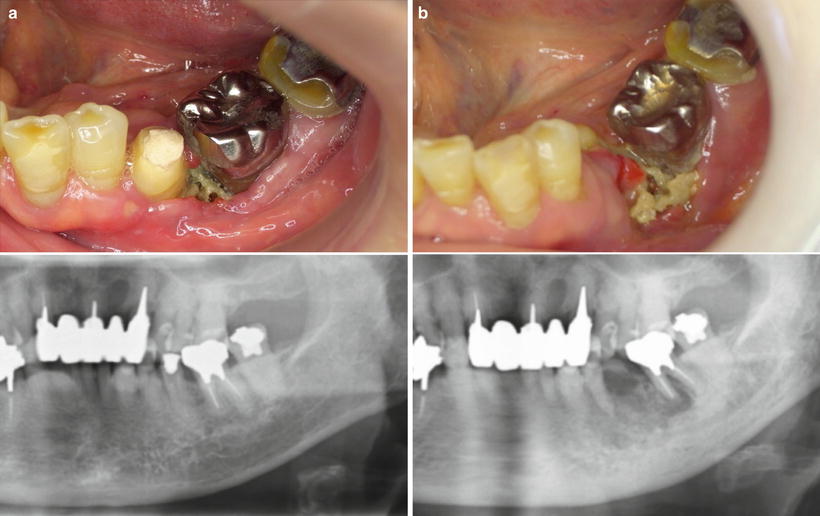

Fig. 14.2
Osteoradionecrosis following radiation therapy. (a) The mandible had been in the field of radiation for the floor of the mouth squamous cell carcinoma. One year after completion of radiation, the necrotic bone had developed. (b) At 3 years, the osteoradionecrosis continued to increase with frequent bouts of swelling, pain, and suppuration. The second premolar exfoliated. Antibiotics were ineffective, and a sequestrectomy was performed
Certain facts emerge from UCSF (University of California, San Francisco) study [21, 24]:
1.
Patients who were edentulous at the time of diagnosis of cancer had a relatively low risk of ORN.
2.
Patients who were dentulous had a greater risk.
3.
The increased risk in dentulous patients appeared to be associated with those who had tooth extractions after radiation therapy.
4.
Dentulous patients with pretreatment extractions or no extractions appeared to have risks similar to those of the edentulous patients.
5.
Spontaneous ORN occurred.
6.
The most important risk factor for the development of osteonecrosis appeared to be the radiation dose to the bone, particularly in the mandible.
7.
Clinical changes in skin and/or mucosa indicating radiation damage were a risk indicator for ORN.
8.
The risk of ORN continued indefinitely following radiation therapy.
Treatment for ORN is variable. The risk of osteomyelitis, an infection of the bone, is increased in the postradiotherapy patient. Analgesic drugs can be taken depending on the severity of pain and patient response. The bony segments that perforate the mucosal tissues create a portal of entry for microbial organisms of the oral flora. If flares of swelling and suppuration occur only occasionally, antibiotics are usually effective. If pain and/or flares occur too frequently or present other difficulties for the patient, surgery must be considered. Aggressive surgical and antibiotic treatment is needed to debride the area and resolve the infection. Sequestrectomy must be done when sequestration develops. Small pieces of necrotic bone can be removed under local anesthesia; on the other hand, larger segments of bone may require hospitalization for their removal. Hyperbaric oxygen (HBO) treatments may help in the regeneration of new blood vessels with a resultant increase in the oxygen supply to the affected bone [25]. The only possible effectiveness is combining HBO with surgery and antibiotics. HBO involves sequential daily exposure to oxygen under pressure (2 h daily in hyperbaric chamber at 2 atmospheres of oxygen, 20–30 times) [19].
14.1.4 Other Oral Complications from Radiotherapy
14.1.4.1 Candida Infection
A resident oral fungal organism with pathogenic capabilities, Candida albicans, causes a common opportunistic infection in the oral tissues of radiotherapy patients. The normal competitive mechanisms among the microbial species of the oral environment and the immunocompetence of the host are usually sufficient to prevent infection of the mucosal tissues by this fungal organism. After radiotherapy, both of these protective mechanisms are altered, which can result in candidiasis in the oral tissues.
The most significant concern is that a Candida infection superimposed over an area of mucositis could be a source of a regional or systemic fungal infection, which could have fatal consequences in a patient already weakened by illness, surgery, radiotherapy, and/or chemotherapy. Treatments for these infections consist of antifungal oral suspensions, such as nystatin, that follow swish-and-swallow protocol [54]. Antifungal troches are difficult to use in patients whose salivary flow has diminished. Patients who wear complete or partial dentures, orthodontic retainers, or night guards must disinfect these appliances in accordance with the manufacturers’ directions. The acrylic portions of these appliances have microscopic porosities in which Candida albicans organisms thrive and re-infect oral tissues that have been cleared of the infection. Systemic fungal infections in these patients have a high mortality rate and must be treated with intravenous antifungal agents in a hospital setting.
14.1.4.2 Loss of Taste
A loss of taste (dysgeusia) may also occur in almost all radiotherapy patients due to damage of the cells which occur primarily in the tongue papilla. There are essentially four tastes: sweet, sour, bitter, and salty. The four primary taste sensations are controlled by different locations on the tongue. For example, the anterior tongue and tip control sweet tastes, and the lateral sides control sour. Bitter tastes are picked up by the circumvallate papillae, and salty tastes can be detected throughout the tongue. The taste bud cells are very sensitive to radiation but usually are capable of repopulating within four months following treatment. Most patients report an alteration in their sense of taste. This is a direct result of the radiation’s effect on taste buds. The extent of damage and the ability to regain the sensation of taste will depend on the cumulative dose of radiation and the number of taste buds involved. The degree to which taste returns is highly variable and varies from patient to patient. When the cumulative dose of 60 Gy has been reached, damage to the taste buds is usually permanent with the sensation of taste being lost [26]. Xerostomia and mucositis also contribute to dysgeusia.
14.1.4.3 Radiation Caries
One result of radiation xerostomia is a pronounced shift toward a highly acidogenic, highly cariogenic oral microflora. The decrease in pH levels, which is commensurate with the amount of damage to the salivary glands, begins the creation of a caries-prone oral environment. When the serous component of saliva decreases and the viscosity increases, adherence of cariogenic bacteria to tooth structure increases. These organisms thrive in the more acidic oral environment that develops after radiotherapy. These factors, coupled with the difficulty that patients have with their oral hygiene maintenance amidst sensitive teeth and soft tissues, create a problem known as “radiation caries” [24]. Radiation-induced dental effects primarily depend on salivary changes and occur when the glands are included in the field of treatment, not on direct irradiation of the teeth themselves. Direct irradiation of teeth may alter the organic or inorganic components in some manner, making them more susceptible to decalcification, but this has not been shown clearly [27].
To prevent or at least minimize radiation caries, oral hygiene must be maximal, including intensive home care and frequent office visits for examination and prophylaxis. Mouth rinsing is essential. Antiseptic mouth rinses, such as chlorhexidine, if tolerated, are helpful in eliminating debris and controlling microbial flora. In patients with hypersensitive mucosa, non-alcohol-containing and unflavored mouth rinses are available. Daily topical fluoride applications, as a rinsing solution, a gel delivered by means of a tray, or brushed on as a paste or gel, are all effective [28].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree