Abstract
Rehabilitation of speech, language, cognitive-linguistic, and swallowing impairments due to brain cancer is facilitated by the speech-language pathologist in a variety of rehabilitative settings. Initial and ongoing assessment through formal and informal evaluation takes into consideration previous level of functioning and factors such as age, level of education, and anticipated discharge goals. Treatment and prognosis are influenced by, among other factors, site of lesion, postoperative changes, and existence of co-morbidities. These factors may be confounded by the effects of radiation and chemotherapy. Favorable outcomes are best achieved through a multidisciplinary team approach. Ongoing research is fundamental to maximizing therapy outcomes and improving quality of life in the care of patients with brain tumors.
Keywords
Aphasia, Apraxia of speech, Brain cancer, Cognitive-linguistic, Dysarthria, Dysphagia, Speech-language pathology, Videofluoroscopic swallow study
Dysphagia in Persons With Brain Cancer
Swallowing is a complex process which involves cortical and subcortical mediation of motor and sensory fibers. Dysphagia, or swallowing impairment, is a variable but not uncommon symptom in patients with brain tumors. The cognitive and sensory-motor impairments that accompany brain tumors, whether pre- or post-treatment, have been shown to have a negative impact on swallowing function. Dysfunction may also occur due to the side effects of radiation, including tissue fibrosis, lymphedema, pain, and xerostomia. Cognitive impairment, a risk factor for dysphagia, may be present as a primary result of the tumor, postsurgical brain edema, or due to the side effects of chemotherapy. Complications of dysphagia include malnutrition, dehydration, and aspiration pneumonia. Dysphagia also has implications for reduced quality of life.
To understand the swallowing deficits that often accompany brain tumor, it is important to have insight into how the normal swallow works. The functional anatomy and physiology involved in swallowing may be represented in four stages which include (1) the anticipatory/pre-oral phase, (2) the oral phase, (3) pharyngeal phase, and (4) esophageal phase. Each phase relies on varying degrees of cognitive, sensory, and motor function. In the anticipatory or pre-oral stage, there must be adequate attention focused on the task of eating or drinking. Cognitive awareness of the food or drink within the environment also excites saliva production. Cranial nerves are activated which will assist in deglutition once food or liquid is introduced into the mouth. During the oral stage, the muscles of the lips, tongue, cheeks, and jaw must effectively accept and remove food from the utensil, as well as be able to move food laterally and medially to facilitate mastication and cohesive bolus formation. The labial and lingual muscles must maintain the food or liquid bolus within the oral cavity both anteriorly and posteriorly until it is ready for transit. Anterior to posterior transit of the bolus is then facilitated in the oral stage by intact lingual and buccal muscles. During the oral phase, the airway remains open and nasal breathing is maintained until the pharyngeal stage begins. Under normal circumstances, the pharyngeal swallow is triggered reflexively when the bolus head passes the line of the mandible (as seen in lateral view radiographically). Once the pharyngeal phase is initiated, velopharyngeal closure must occur to prevent nasal regurgitation and timely closure of the laryngeal vestibule must occur to ensure effective airway protection. Retraction of the base of tongue, hyolaryngeal elevation and anterior movement, epiglottic retroflexion, pharyngeal wall contraction, as well as relaxation and opening of the upper esophageal sphincter (UES) effect pressure on the bolus, propelling it into the cervical esophagus. Adequate timing and duration of the UES opening allows the bolus to clear from the hypopharynx at which time breathing may resume via the mouth or nasal passages.
Assessment of swallowing function may be initiated via a clinical swallowing evaluation (CSE). This typically involves a cranial nerve assessment focused on CN V, VII, IX, X, and XII which play a significant role in oral and pharyngeal swallow function. The CSE will also note how the patient presents in terms of level of arousal and overall mentation, respiratory status, vocal quality, oral awareness of food/utensil, oral management of the bolus, as well as overt signs suggestive of pharyngeal dysfunction. For example, on a bedside examination, multiple swallows may be indicative of pharyngeal residue; throat clearing, cough, and wet vocal and/or breath sounds may be suggestive of laryngeal penetration or aspiration. When the CSE is not conclusive, an objective instrumental assessment may be indicated. The modified barium swallow study (MBS) and the flexible endoscopic evaluation of swallow (FEES) are both considered gold standard tools for the assessment of oropharyngeal dysphagia. The MBS and FEES are helpful in determining presence, absence, and risk for aspiration. If there is increased risk for aspiration, effectiveness of compensatory swallow strategies can be evaluated. Information can be obtained as to whether positional changes or diet modification may improve airway protection. The MBS and FEES are able to reveal specific aspects of dysfunction that are not evident during the clinical bedside examination. Among other findings, silent aspiration, unilateral pharyngeal weakness, pharyngeal residue, incomplete or absent epiglottic retroflexion, UES dysfunction, and pharyngoesophageal backflow of food or liquids may be suspected, however not definitively identified unless visualized fluoroscopically or endoscopically. Additionally, FEES can provide useful information in regard to secretion management, condition of mucosa, and structure and function of the vocal folds. These objective studies are often essential in directing therapy goals and in determining reliable measures of improvement.
Oral, pharyngeal, and pharyngoesophageal dysphagia can be caused by reduced motor control of muscles and structures, reduced sensation, as well as reduced processing of sensation. Bilateral or unilateral labial, lingual, and buccal weakness can result in poor management of food or liquid in the mouth resulting in oral dysphagia. Drooling may also occur. Spillage of material either out of the mouth anteriorly or prematurely to the oro and hypopharynx can occur from reduced strength and sensation of labial and lingual structures. Reduced buccal strength or sensation can result in pocketing of material in the lateral and anterior sulci. Reduced sensation in the pharynx can cause the pharyngeal swallow reflex to be delayed, thus leaving the airway exposed to penetration or aspiration of food and liquid as the material advances before airway closure is initiated. Weak base of tongue movement, reduced pharyngeal wall contractility, and reduced hyolaryngeal movement can result in significant pharyngeal residue which may be susceptible to entering the airway.
Although dysphagia can occur in individuals with cortical and subcortical lesions, those with brainstem lesions often present with significant dysphagia due to the fact that the central pattern generator for swallowing is located in the medulla. The pons acts as a relay station for sensory nerve impulses from oral, pharyngeal, and laryngeal receptors. The pons contains the nuclei of cranial nerves V (trigeminal) and VII (facial), whereas the medulla houses the nuclei of CN IX-XII. Because of impact to the glossopharyngeal (IX), vagus (X), and hypoglossal nerves (XII), lesions localized to the medulla may result in relatively normal oral function in the setting of a profoundly impaired pharyngeal swallow. Studies also indicate high risk for dysphagia along with its sequela of malnutrition, dehydration, and aspiration following surgery, particularly for posterior fossa tumors in both children and adults.
Dysphagia may occur due to the side effects of radiation. Tissue fibrosis, lymphedema, pain, and xerostomia can limit normal movement of the oral, pharyngeal, and laryngeal structures and can impact patients’ ability to obtain adequate nutrition and hydration. Providing medications via oral route can also be challenging.
Cognitive impairment may be present as a primary result of brain tumor, postsurgical brain edema, or due to the side effects of chemotherapy. Impaired cognition is generally correlated with oral stage dysfunction due to cortical involvement and can result in reduced attention to the feeding process, reduced awareness of food/liquid in the mouth, inadequate mastication and/or oral bolus formation, and inadequate oral control of food or liquid with potential for premature spillage over the base of the tongue. Agnosia, reduced attention, reduced insight into deficits, and overall reduced executive functioning can put patients at risk for inadequate nutrition and hydration and may increase risk for aspiration.
Various rating scales, such as the MD Anderson Dysphagia Inventory (MDADI) and the Swallowing Quality of Life questionnaire (SWAL-QOL), allow for a subjective evaluation of the impact of dysphagia on quality of life for cancer patients. Although there is evidence supporting validity of these rating scales, some researchers have found that the degree to which patients reported swallowing difficulties was not necessarily indicative of the actual level of impairment for patients with primary brain tumors, and thus was not predictive of risk for aspiration or risk for inadequate nutritional intake. These findings highlight the importance of thorough clinical assessment through the use of subjective and objective swallowing evaluations.
Incidence of dysphagia and response to rehabilitation in patients with brain tumor has been found to be similar to that of patients with stroke. In a retrospective study, Park et al. found that swallowing dysfunction was comparable in brain tumor and stroke patients and that lesion location was a determining factor in dysphagia. Dysphagia was noted in 72.5% of patients with brain tumor versus 77.5% of stroke patients, and when lesion location was taken into account, findings supported increased risk for dysphagia in patients with infratentorial lesions (90.5%) versus those with supratentorial lesions (52.6%). Vallecular and pyriform residue was a more frequent finding in patients with infratentorial lesions as was lower scores on the ASHA NOMS rating scale. More restrictive diet consistencies and higher supervision levels were also indicated for patients with infratentorial lesions.
Evidence suggests that rehabilitation efforts comparable to those provided for stroke patients will benefit brain tumor patients as well. There is expectation of some initial spontaneous recovery after medical management followed by the benefit of rehabilitative services lasting anywhere from several weeks to several months. Patients with tumors of a progressive nature or frequent recurrence (e.g., glioblastomas) may warrant a greater focus on maximizing independence through the use of compensatory techniques; however, improvement in productivity and quality of life as a result of therapy may occur despite the patient’s poor prognosis. Repeat objective studies may be indicated in keeping with disease progression and recommendation for alternative means of nutrition, hydration, and medications may warrant either placement of nasogastric or percutaneous gastrostomy tube. Palliative or hospice considerations may be appropriate and will impact recommendations for diet consistencies as well as oral versus nonoral means.
Dysphagia, especially in the acute stages, can often be remediated through the use of compensatory strategies and/or modifications to diet consistency. Depending on the specific deficit, risk for aspiration can often be reduced with postural changes such as chin tuck positioning or head rotation. Maneuvers can be employed to improve airway protection, reduce pharyngeal residue, or increase extent and duration of UES opening (see Fig. 7.1 ). Safety and efficiency of swallow can also be improved with the use of thickened liquids or modified food textures such as purees. Although compensatory techniques are often effective in managing immediate concerns, actual rehabilitation of the swallow may be more effectively achieved through active exercise. Traditionally, dysphagia therapy has focused on strength building, which may be appropriate if weakness is the cause of the disordered swallow pattern. If components of apraxia or spasticity exist, then a focus solely on strength building will be a misguided approach. Evidence-based practice suggests that rehabilitation of the swallow may be most effective if it follows the principles of exercise science with intensity and specificity of task designed to reach functional goals. In much the same way a physical therapist may work with a patient to improve their mobility, building coordination, speed, and endurance to facilitate functional motor movement in swallowing, which may be as important as building strength.
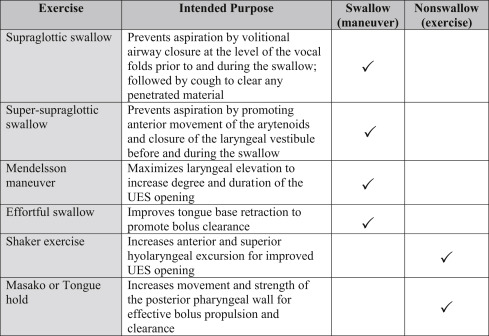
Leading researchers in the field of speech pathology have questioned whether many commonly used dysphagia exercises currently have an adequate evidence base to support long-term efficacy. In a critique of the literature, Langmore and Pisegna found that one out of five “swallow exercises” and two out of four “nonswallow exercises” had evidence of long-term efficacy for treatment of dysphagia. According to the critique, adequate evidence existed only for the Mendelsohn maneuver, the Shaker head-lift exercise, and expiratory muscle strength training. Swallow and nonswallow exercises may be further defined as maneuvers (which incorporate active swallowing) versus exercises (which do not) ( Fig. 7.1 ). Some techniques have both compensatory and rehabilitative value. The supraglottic and super-supraglottic maneuvers can be used during oral intake to prevent aspiration and can also be used as part of an exercise regimen to produce lasting improvement in airway protection.
The principles of exercise science suggest that the reacquisition of a functional swallow should include three essential components of motor learning: practice in the specific task, progressively increasing difficulty, and the inclusion of feedback. Whether visual, verbal, or tactile, and additionally whether it is intrinsic or extrinsic, feedback is necessary to promote attempts at correction on repeated trials. Neuromuscular electrical stimulation (NMES) is often employed to improve oral, pharyngeal, and laryngeal responses during intensive and repetitive swallowing drills. Therapy incorporating this modality typically meets the motor learning requirements of overload and specificity. Recent studies show significant gains in swallowing during therapy with NMES, although evidence supporting efficacy of NMES versus traditional therapy or other techniques alone continues to be mixed.
The McNeill Dysphagia Therapy Program offers skill-based training protocols that incorporate the concepts of task specificity and task challenge. There is increasing support for the use of a feedback modality such as surface electromyography (sEMG) in conjunction with targeted skill-based training in order to achieve functional gains in swallowing. It should be noted that newer NMES devices do in fact have integrated sEMG capabilities. Ongoing research is needed to develop more efficacious therapy techniques. Speech pathologists, in collaboration with multidisciplinary teams, must incorporate current evidence with their clinical expertise in order to develop individualized plans of care that take into consideration patient care needs and values. The specific physiologic impairments of the patient and goals of care should dictate the particular swallowing interventions provided.
Motor-Speech Impairments in Persons With Brain Cancer
Speech is comprised of the particular articulatory movements required to produce intelligible phonemes, or individual sounds, as well as the subsystems which allow for each sound to be created. To produce an intelligible sound, the patient must first be able to achieve adequate respiratory support and coordination by inhaling and exhaling with sufficient tidal volume. The patient must then be able to plan and execute the movements of muscles associated with speech articulators (i.e., lips, tongue, jaw, cheeks, velum). Injury secondary to brain tumors, site dependent, as well as the medical treatment of brain tumors may result in two forms of speech disorders: acquired apraxia of speech (AOS) and dysarthria.
AOS is a “neurologic speech disorder that reflects an impaired capacity to plan or program sensorimotor commands necessary for directing movements that result in phonetically and prosodically normal speech.” Site of lesion for AOS typically involves the motor cortex and supplementary motor area. It is not caused by weakness, rather the inability to effectively plan and execute motor movement.
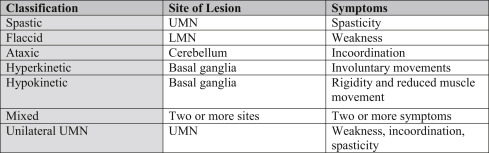
Dysarthria is a motor speech disorder wherein a patient’s articulatory muscles are too weak or have been surgically removed resulting in the inability to produce normal speech. Conditions causing dysarthria include the atrophy, fibrosis, edema, surgical removal, and reduced sensation of musculature involving the lips, tongue, jaw, cheeks, and velum. Dysarthria could also be caused by medications which act on the “central nervous system, such as narcotics, phenytoin, or carbamazepine.” There are seven different types of dysarthria (see Fig. 7.2 ).
Both AOS and dysarthria impact a patient’s ability to communicate effectively, which may negatively influence patient safety and quality of life. Reduced speech intelligibility minimizes the response acuity necessary for relaying important information between patient and caregiver (i.e., pain, personal history). It also impacts the patient’s quality of life (i.e., social interaction, attitude toward engaging in therapy). The speech-language pathologist will assess for AOS and dysarthria utilizing both a formal and informal evaluation to create a personalized plan of care and provide education to both the patient and patient’s family.
Causation of AOS and dysarthria is the primary indicator of treatment and prognosis. Individual plans of care with be tailored to restorative or supportive outcome measures dependent upon whether the speech disorder was caused by a change in tissue integrity or the removal of tissue. Current practice utilizes oral motor exercises (OMEs) as a restorative means to treat atrophy resulting in slurred or imprecise articulation. Research has provided evidence that OMEs do increase muscular strength when compared with those who did not do the exercises. However, there is ongoing criticism of OMEs, specifically detraining effects post discontinuation and that “nonspeech OMEs often do not adhere to accepted principles of muscle training.” OMEs are comprised of the following movements, either against resistance or in sets of repetitions: buccal, labial retraction/protrusion, labial lateralization, cheek puff, alternating cheek puff, lip press, lingual excursion, lingual lateralization, lingual elevation/depression, lingual-palatal sweep. Ultimately, greater research into strength training of particular orofacial musculature is needed.
Tactile-kinesthetic speech motor treatment [e.g., prompts for reconstructive oral muscular phonetic targets (PROMPT)] is a current practice utilized to treat motor planning disorders resulting in AOS. Research is finding positive outcomes for patients who utilized PROMPT for improving “speech movements in utterances of varying linguistic complexity.” However, limited research has been completed with patients specifically diagnosed with brain tumor.
A supportive therapeutic approach will be taken during an inpatient rehabilitation stay if permanent damage is incurred as the result of surgery or nerve impingement from a nonoperative tumor left intact. Supportive approaches include augmentative and alternative communication (AAC) devices (i.e., communication boards, tablets, computers). The inpatient speech-language pathologist’s role is to assess the patient’s ability to utilize a simple communication board and create an appropriate and functional board tailored to the individual while providing training for functional use. Simple boards are often comprised of basic elements, highlighting basic wants and needs (i.e., pain, site of pain, bathroom, hunger/thirst, etc.). If functional speech cannot be improved, outpatient therapy may address high-tech augmentative devices to facilitate communication. Communication boards can be effective in conveying simple, basic needs; however, they are often limited as to what can be expressed and in the timeliness of expression.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree