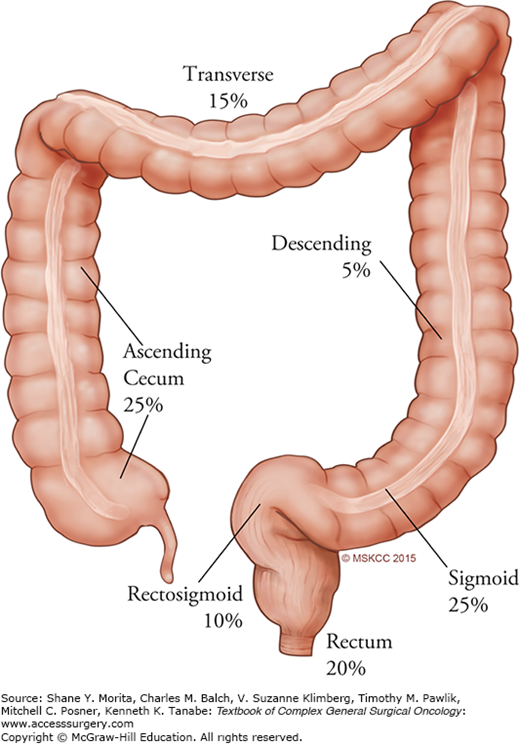
Complete surgical resection of a primary non-metastatic colon cancer is the mainstay of treatment and holds the best chance for potential cure. The relative frequency of colorectal cancer by anatomic site of origin is shown in Fig. 108-1. Adequate resection involves removal of the involved segment of large bowel, mesentery, and associated vascular supply to completely excise the lymphatics, which harbor metastatic disease. At least 12 lymph nodes should be removed and pathologically evaluated to determine stage.1 Laparoscopic colectomy after diagnosis of colon cancer is now an accepted approach that is not inferior to conventional open surgical resection. The Clinical Outcomes of Surgical Therapy (COST) trial randomized 827 patients with colon cancer to open or laparoscopic operations, noting no significant differences in overall survival.2 The data show that patients in the laparoscopic group recovered faster, used less pain medications, and had no more short-term morbidity or mortality compared with the open group.
Robotic surgery has come to the forefront as a modality that improves upon the mechanical disadvantages of laparoscopic surgical techniques. In recent work, a meta-analysis reviewing 39 case series, 29 comparative studies, and one randomized trial indicates that robot-assisted colorectal surgery is safe and feasible when compared with laparoscopic and open surgery. The oncologic outcomes have been similar in the robot-assisted surgery groups. Less blood loss, shorter hospital stay, fewer conversions, and lower complication rates were noted when compared with laparoscopic or open resections.3
The American Society of Colon and Rectal Surgeons (ASCRS) has put forth a set of practice parameters for the management of colon cancer, based on grades of evidence,4 in support of specific preoperative assessment and treatment strategies. Guidelines put forth by both the National Comprehensive Cancer Network (NCCN) and the American Society for Clinical Oncology (ASCO) are also important in determining optimal delivery of best care to patients.5 Here, we will discuss the existing strong treatment recommendations in settings, where benefits clearly outweigh risk (grade 1A to 1C). It is beyond the scope of this chapter to explore the details for each topic, but the general recommendations are highlighted.
To begin, a thorough history and physical examination and routine laboratory analysis, including CEA, should be part of the initial work-up of patients with colon cancer. Additionally, every patient should undergo a full colonic evaluation, with histologic analysis of the colon lesion, prior to initiation of treatment. If colonoscopy is incomplete, then short interval postoperative (3-month) colonoscopy should be performed. Radiologic staging should also be part of routine preoperative work-up. Staging of the disease should be done in accordance with the American Joint Committee on Cancer (AJCC)/Tumor Node Metastasis (TNM) system.
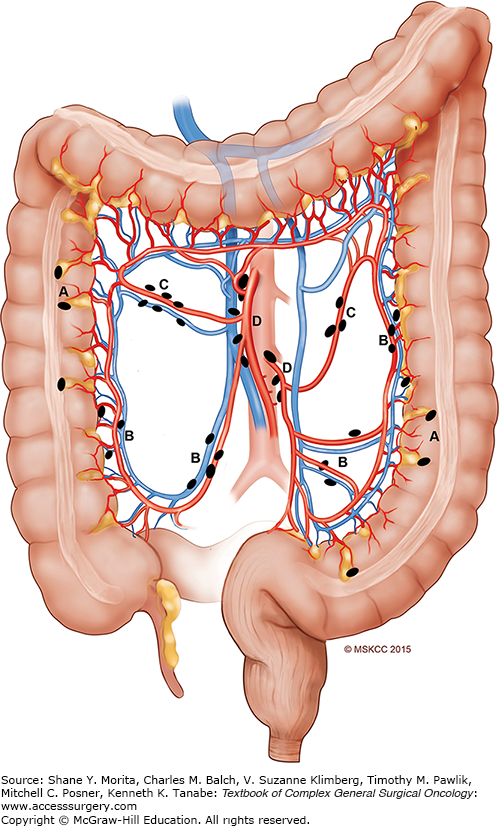
From an operative standpoint, a thorough exploration should be performed and documented; to achieve a complete lymphadenectomy, the extent of resection must correspond to the associated lymphovascular drainage of the specific site of colon cancer (see Fig. 108-2). Sentinel lymph node mapping cannot be recommended as a replacement for standard lymphadenectomy. Involved adjacent organs should be resected en bloc. Synchronous colon cancers can be treated by separate resections, or, alternatively, with subtotal colectomy. In the female patient, routine oophorectomy is not necessary unless there is gross evidence of tumor extension. It has been demonstrated that laparoscopic and open colectomy result in equivalent oncologic outcomes for localized colon cancer; however, these results are surgeon-specific, based on specialty training. Malignant polyps should first be individually managed by determining morphology and histology, as 2% to 5% of these polyps harbor an invasive malignancy. If a patient presents with perforation, the surgeon must address the perforation and the tumor should be resected following basic oncologic principles, with the patient’s safety and survival of the acute event being paramount. Patients presenting with obstruction are ideally treated with definitive surgical resection with a primary anastomosis. A complete surgical report should be available for each patient, and should include the diagnostic work-up, intraoperative findings, and technical details of the specific procedure.
In the adjuvant setting, NCCN, ASCO, and ASCRS all recommended adjuvant chemotherapy delivery to patients with stage III colon cancer. Controversy remains regarding the use of adjuvant therapy for stage II patients, and a medical oncology consultation is advised. New molecular prognostic markers are emerging,6,7 and may provide better evidence in support of adjuvant therapy in select stage II patients.8
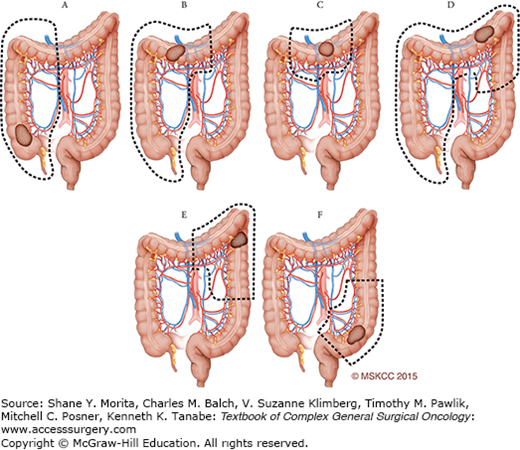
Approaching an operation for colon cancer is guided by the principle of curative-intent surgery in order to remove the primary tumor with adequate margins and sufficient lymph nodes for pathologic evaluation. A basic guideline for operative approach according to the site of the tumor is shown in Fig. 108-3 (e.g., ascending colon mass, hepatic flexure or hindgut tumors). Many guidelines and approaches exist for the technical performance of each segmental colonic resection, and general principles will be covered below along with the standard anatomic landmarks and operative steps common to the conduct of a colon cancer operation for an individual patient. We will cover right and left colectomy in detail for both minimally invasive and open approaches. We will provide a general framework for the indications and approach for total abdominal colectomy with ileorectal anastomosis.
The true extent of lymphadenectomy is based on careful understanding of the anatomy and the pattern of spread in intestinal cancers. Regional lymphatics of the colon are well described9 (see Fig. 108-2). In the current TNM staging system for colon cancer, the presence of metastatic colon cancer in the regional mesenteric lymph nodes is the primary determinant for use of adjuvant chemotherapy. Several retrospective single institution studies have demonstrated an association between increased numbers of examined lymph nodes and the presence of lymph node metastasis (LNM).10,11 Furthermore, an analysis of the National Cancer Database (NCDB) found that patient outcomes after treatment for T3N0 colon cancer can be stratified by the quantity of lymph nodes retrieved.1
Because of these and other studies, many national associations, including the College of American Pathologists and the Standards Practice Task Force of the ASCRS, have advocated for establishing an acceptable minimum of 12 to 15 lymph nodes.12,13 Of note, lymph node yield during colon cancer surgery is being recommended as a benchmark of quality care. The National Quality Forum (NQF) on consensus standards for quality of cancer care, in collaboration with the American College of Surgeons (ACS), the American Society for Clinical Oncology (ASCO), and the NCCN, endorses two “accountability measures, [which] can be used for such purposes as public reporting, payment incentive programs, and the selection of providers by consumers, health plans, or purchasers.” These measures are the use of adjuvant chemotherapy for stage III colorectal cancer and a 12 lymph node minimum to determine nodal status accurately.
Despite these national guidelines, many hospitals fail to meet these standards. For example, only 78% of NCI-designated Comprehensive Cancer Centers, 52% of other academic hospitals, 53% of Veterans’ Administration hospitals, and 34% of community hospitals are compliant.14 This issue has gained national media attention15; however, some recent analyses have rejected the idea that a fixed minimum value should be set. Tekkis et al16 performed a multicenter review of locoregional colorectal cancer and demonstrated that many factors beyond the control of surgeons and pathologists were associated with lymph node yields, including tumor differentiation, use of neoadjuvant RT, patient age and ASA, and cancer stage. Furthermore, analysis of SEER data (1995 to 2005) found that the number of lymph nodes examined following colon resection was not associated with use of adjuvant chemotherapy, stage, or survival.17
In an effort to provide more granular data on lymph node yields, our group performed a retrospective analysis of a prospective database of 152 Memorial Sloan Kettering Cancer Center (MSKCC) patients with detailed anatomic data.18 We expect that certain variables have been previously associated with lymph node yields for reasons other than tumor biology. For example, patient general health may be a confounder as it may influence surgical decision-making regarding the extent of mesenteric resection. Other variables such as the presence of LNM may be confounders of lymph node yield because it decreases the need for the pathologist to find additional lymph nodes. We noted that tumor size, location, pedicle resection number, and tattoo use were associated with a significant linear relationship with lymph node yield when controlling for multiple factors including age, gender, tumor stage, tumor size tumor location, specimen length, lymphovascular invasion, or perineural invasion and differentiation.
Improvement in lymph node yield and subsequent improvement in outcomes have been proposed by complete mesocolic excision (CME) in the mesocolic plane with central vascular ligation.19 The goal is to remove the entirety of the mesocolon along embryologic tissue planes with transection of the critical vasculature at the origin. A group in Erlangen, Germany has routinely practiced this technique, and it reports excellent 5-year survival rates of >85%.20 Adherence to these principles does require detailed knowledge of anatomical planes21 and avoidance of any breaches of visceral fascial layers, which theoretically could lead to tumor cells contamination of the peritoneum and metastases. Additionally, work from Japan has called for a D3 lymphadenectomy along with CME to further improve outcomes in keeping with resecting at least 10 cm proximal and distal to the tumor edge (pericolic lymphnode metastases beyond 10 cm are quite rare22). D3 lymphadectomy is defined as skeletonizing and ligating the vasculature flush with the origin of the involved vessels (e.g., ileocolic) followed by en bloc removal of the lymphatics along the lateral and ventral bit of the SMV to Henle’s gastrocolic trunk and final transection of the involved distal vasculature (e.g., right colic and right branch of middle colic vessels) at the respective origins.23 Interestingly, resection of the longitudinal bowel wall within 10 cm of the tumor is becoming a “benchmark” for CME in some centers in Japan and Europe24 while the D3 dissection has been a more contentious issue (especially in western circles). However, recent work has demonstrated that the extra effort to obtain pericolic lymph nodes 10 cm from the tumor (e.g., N3) was associated with possible improvement in 5-year DFS.25 The principles of CME seem sound, and our group and others have been moving toward CME for routine colectomy using both MIS and open approaches as appropriate for each patient. Evaluation of larger subsets of patients evaluated compared to historical controls is needed to further support this approach as a randomized trial would likely be unwieldy and difficult for patients to consent to such a trial.
The impact of hospital volume and surgical outcomes has been investigated extensively in the recent literature. A recent study utilized a nationwide inpatient sample database of 54,000 patients treated from 2003 to 2007 to evaluate whether or not surgeon volume plays a role in morbidity and mortality in patients undergoing elective resection for colon cancer. Upon adjusting for multiple covariates including hospital volume, high-volume surgeons had an associated reduction in morbidity and mortality. The lowest levels of mortality were noted for high-volume surgeons working in high-volume hospitals. Patients undergoing resection by surgeons who performed at least 10 colon cancer operations annually had a 25% lower risk of death, compared with patients undergoing resection by surgeons who performed four or fewer procedures per year. Multiple studies have demonstrated that high-volume surgeons achieve a lower mortality rate, and that surgeon volume is a predictor of outcome following primary resection for colon cancer.
Another study on surgeon volume utilized data from the University Health System Consortium from 2008 to 2011 to examine usage of laparoscopy as well as cost and outcomes in a cohort of 17,749 patients undergoing colon cancer resection. Laparoscopy in association with surgeon volume and secondary outcomes such as complications, length of stay (LOS), and total costs were assessed. High volume was defined as >11 colectomies per year, and low volume as <5 per year. The study showed an overall increase in utilization of laparoscopy for high-volume surgeons over the study period. Total costs were found to be ~$1500 less for high-volume surgeons, and patients operated on by these surgeons had 25% fewer complications. Of note, the chances of requiring a reoperation was 30% less for patients treated by high-volume surgeons.
Similar results have been demonstrated in robot-assisted colorectal surgery. An analysis of a national inpatient database of 1428 cases, across 123 hospitals and including 411 surgeons, demonstrated that, as the volume of cases increased, rates of overall complications, LOS, and cost significantly decreased. Furthermore, high-volume hospitals and surgeons were associated with significantly lower rates of ileus and bleeding. These data indicate that the use of robotic surgery should be limited to high-volume surgeons and hospitals, so that the highest quality outcomes can be achieved at lower cost and in less time.
The most common hospital-acquired infection is a surgical site infection (SSI). SSIs are associated with major costs to the patient and healthcare economy. SSIs have the potential to result in more than $1.6 billion in direct costs. Adherence to SSI reduction programs is an important step toward reducing costs. At the Mayo Clinic, using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) to identify SSI-associated factors, an SSI-reduction bundle was implemented, which reduced SSIs in a short period of time. With a goal of reducing SSI by 50%, the group established a clinical bundle including preoperative, intraoperative, and postoperative processes to address patient factors, Surgical Care Improvement Project (SCIP) measures, facility practices, and postsurgical recognition of SSIs. Their SSI rate for the two years prior was 9.8%; after implementation of the bundle, the SSI rate declined to 4% overall. This study demonstrates that a multidisciplinary team can programmatically implement strategies resulting in impact on patient SSI outcomes, along with a reduction in associated costs. Other strategies to reduce SSIs include preoperative oral antibiotic regimens. These regimens are associated with reduction in LOS and fewer 30-day readmissions for infectious causes, as demonstrated by a study using Veterans Affairs Administration and Pharmacy Benefits Management data on >8000 patients undergoing elective colorectal resection from 2005 to 2009. The practice of administering an oral antibiotic regimen before colorectal surgery has existed for a long time, and should be carefully considered on an institutional level as part of the treatment bundle aimed at reducing SSIs. Recent analysis on preoperative bowel preparation and postoperative complications in colon surgery indicated a significant decrease in postoperative morbidity with the use of mechanical and oral antibiotic preparations in 5021 patients undergoing elective colon resection from the NSQIP database,26 and we adhere to this practice at MSKCC.
Quality of life (QOL) factors measured at baseline, including preoperative mood rating and emotional well-being, have been noted as independent predictors of long-term survival. In a study of 117 patients, better mood predicted better survival and worse emotional well-being predicted worse survival, highlighting the notion that baseline QOL parameters may be associated with survival in non-metastatic colorectal cancer patients. Notably, there is even some data to suggest a psycho-neuro-immunological effect on tumor biology via release of proinflammatory cytokines.
Anastomotic leaks after colorectal resections are a significant source of postoperative morbidity, potential mortality, and prolonged hospital stay. A recent study evaluated QOL in patients undergoing a low anterior resection complicated by a leak (n = 25), compared with patients who did not suffer a leak postoperatively (n = 50). Not surprisingly, leak patients were in the hospital longer and had a diverting ostomy for a longer time. Physical, emotional, and social function and related overall QOL was significantly decreased in the leak group. Although this was a small study, it is apparent that anastomotic leaks tax the patient, with associated prolongation of hospital stay and increased use of available resources.
A malignant polyp is an adenoma in which a focus of invasive carcinoma is found in a polyp usually removed by snare during colonoscopy. Without invading the muscularis mucosa, a tumor has no metastatic potential because of the absence of lymphatics in the colonic lamina propria. The likelihood of invasive disease arising in a colorectal polyp is associated with morphology, histology, and size of the lesion. Polyps can be classified as tubular, villous, or tubulovillous. The incidence of invasive malignancy differs markedly for these three histologic subtypes of colon polyps: lowest in adenomatous polyps, intermediate in tubulovillous adenomas, and highest in villous lesions. Polyp size is directly correlated with the presence of dysplasia or malignancy. The relative risk of adenocarcinoma being present in a polyp is strongly related to the size of the polyp.27 Similarly, the presence of invasive cancer on complete polypectomy can be predicted based on the degree of dysplasia.28 Endoscopic polypectomy is curative in the vast majority of cases of pedunculated polyps. When the focus of malignancy is confined to the head of the polyp and on histological examination, the carcinoma is superficial with clear margins and no high-risk features (e.g., no lymphovascular invasion, perineural invasion, or poorly differentiated histology); post-polypectomy follow-up should include close observation without formal colectomy. However, incomplete resection, poor differentiation, and lymphatic or vascular invasion are associated with lower cure rates following endoscopic polypectomy; therefore, segmental colectomy is usually indicated.
The treatment of malignant colonic polyps is primarily dictated by the risk of metastasis to the mesenteric lymph nodes. It has been estimated that 8.5% to 25% of polyps harboring invasive carcinoma will metastasize to regional lymph nodes. The probability that regional lymph nodes will be involved with cancer is related to certain pathologic features of malignant colorectal polyps such as poor differentiation, vascular or lymphatic invasion, invasion below the submucosa, and positive resection margin. Generally, the presence of one or more of these features is an indication for resection. Among these factors, depth of invasion is the most important prognostic factor for mesenteric lymph node involvement with malignant polyps as has been characterized by Haggitt et al.29
Another indication for colectomy is benign polyps that are not amenable to endoscopic resection due to size or sessile nature. The rationale for such an aggressive surgical approach in treating a benign lesion is twofold. The first reason is the possibility that a polyp may harbor a malignancy that cannot be completely excluded based on the results of endoscopic biopsy. Although such a possibility depends mainly on the size and histology of the polyp, it is impossible to definitively declare a polyp as “benign.” Results from studies evaluating the ability of endoscopic biopsy to diagnose malignancy have shown that the technique is only 81% to 84% sensitive.30,31 Therefore, a surgical resection for an unresectable polyp is indicated. The second reason for an aggressive surgical approach to colonic polyps is the documented propensity of polyps to grow and subsequently undergo malignant degeneration.32
The clinical decision to perform a colectomy for a colonic polyp should be made on a case-by-case basis. Factors to consider should include the histologic characteristics of the polyp and the patient’s operative risk and preferences. In addition, more widespread adoption of newer techniques such as endoscopic mucosal resection and endoscopic submucosal dissection may obviate the need for surgery in a subset of patients with large polyps and no evidence of invasive cancer.
Finally, the presence of colonic polyps in a patient who is already diagnosed with colon carcinoma is another dilemma that is worth mentioning. It has been estimated that approximately 7% of patients with polyps harbor a synchronous colorectal carcinoma, but about 29.7% of patients with carcinoma have synchronous polyps.33 Most synchronous polyps are identified on preoperative colonoscopy, and the colon can often be cleared of these lesions before operation. As a general rule, if the polyps are confined to the same anatomical region as the index carcinoma, a formal segmental resection is indicated. If the polyps are at different anatomical sites, a colonoscopic excision of these polyps should be attempted first. If one of the excised polyps contains a carcinoma or if the polyps are not suitable for colonoscopic resection, one option is to perform multiple segmental resections with multiple anastomoses. The other option is a subtotal or even total colectomy to include the polyps and the index malignancy.34
Surgical options in this setting can be both prophylactic and therapeutic (more details in the final section of the chapter). In a familial adenomatous polyposis (FAP) patient, the most common surgical interventions are total abdominal colectomy with ileorectal anastomosis and total proctocolectomy with either an ileal pouch-anal anastomosis (TPC with IPAA) or an end ileostomy. The ileorectal anastomosis option is reserved for those whose rectal segment has minimal disease and can be surveyed and controlled endoscopically. The advantages of this approach are less morbidity (e.g., bladder and sexual function preserved, relatively normal bowel function). Obviously the rectum is at risk with a range of rectal cancer occurrence from 10% to 50% with more than half of the patients eventually requiring a rectal resection.35 The advantage of TPC with IPAA includes elimination of risk but there is a trade-off due to the complexity of the procedure and associated bladder, bowel, and sexual dysfunction. An end ileostomy is a good option for those with advanced cancer or those patients not willing to take on the risks of a TPC with an IPAA.
Surgical management of Lynch syndrome patients is individualized in our hands. For patients presenting with cancer or polyps not amenable to endoscopic removal, a total abdominal colectomy with ileorectal anastomosis can be considered. Other options are segmental resections and close endoscopic surveillance and consideration for chemoprevention options. Also, women should consider a total abdominal hysterectomy with bilateral salpingo-oophorectomy if child-bearing is completed due to the risk of endometrial cancer. If a Lynch patient presents with rectal cancer, then a TPC with IPAA is likely the best option with a segmental resection being less preferable for those who are risk averse. See the chapter on hereditary syndromes for more details on hereditary bowel cancer treatments.
Obviously after a portion of a hollow organ is resected, it must be reconnected to maintain continuity and enable passage of fecal content. Furthermore, the ability to perform an anastomosis allows the surgeon to remove the pathologic lesion and to potentially avoid a colostomy or an ileostomy. Multiple configurations can be used: end-to-end, end-to-side, or side-to-side. End-to-end is appropriate for equal luminal diameters on either end, end-to-side anastomoses for luminal disparities on either side, or an end-to-side can be used if a larger anastomosis is needed in the setting of a narrowed lumen.36 A long debated topic on the superiority of hand-sewn versus stapled anastomoses has been tested in numerous randomized controlled trials. In a recent Cochrane Review of 1233 patients with 622 undergoing a stapled anastomosis and 611 undergoing a handsewn anastomosis, the safety and efficacy were evaluated by meta-analysis looking for differences between the two (e.g., mortality, anastomotic dehiscence, stricture, hemorrhage, need for reoperation, wound infection, anastomosis time, or hospital stay).37 No statistically significant differences were noted, except that stricture was more common in the stapled setting (p<0.05) and not surprisingly the handsewn technique took longer. Most colonic anastomoses in our hands are done in the stapled fashion (functional end-to-end anastomosis with a GIA stapler and a TA stapler to close the common enterotomy); however, with the advent of laparoscopic or robotic-assisted technology, use of an isoperistaltic side-to-side anastomosis combining stapling and subsequent suturing of the common channel defect is becoming more promising and time efficient.
All patients undergoing colectomy of any type at our institution undergo oral mechanical bowel preparation with a clear liquid diet and a polyethylene glycol-based agent, in combination with oral antibiotics, on the day prior to surgery. We use epidural catheters placed in the preoperative holding area for open segmental colectomies to aid in postoperative mobility, pain control, and a generally expeditious recovery. We perform TAP blocks on the minimally invasive segmental colectomies either preoperatively or intraoperatively (surgeon preference). We follow early recovery preoperative principles. Before induction of anesthesia, a prophylactic dose of low-molecular-weight heparin and antibiotics is administered. An intravenous dose of a broad-spectrum antibiotic covering gram-negative bacteria and anaerobes is delivered. An orogastric tube is placed at the discretion of the anesthesiologist or the surgeon. Sequential compression devices are placed bilaterally unless contraindicated, and appropriate padding and straps are placed to maintain patient security no matter the positioning of the table during the course of the operation. The patient is covered with a body warmer to prevent hypothermia. A Foley catheter is placed under sterile conditions to measure urine output. For minimally invasive colectomies, we routinely place a large foam mat under the patient to prevent sliding when changing the position of the operating bed. The upper chest is secured with a Velcro strap and tested.
The operating room should be spacious enough to allow sufficient room for the surgeon, assistant, scrub nurse, patient cart, robot console (if applicable), camera tower, and CO2 insufflation machine. After intubation and placement of a urinary catheter, orogastric tube, and IV access, the patient is positioned supine with the iliac crest centered over the flexion point joint (break) of the table. The patient’s arms are tucked alongside the body and padded to lessen the possibility of brachial plexus injury. This allows for easier docking of the robot (if applicable) and provides extra space for the assistant at bedside. Pressure points and bony prominences are padded, and the body is secured to the operating table with straps around the legs and shoulders. We also prefer to use anti-skid foam cushion in order to avoid the patient sliding with changes in position. If needed, the table can be flexed 10° to 15° at the break to lower the patient’s legs, in order to prevent external collisions with the robotic arms after docking. Final table adjustments should be made prior to draping, and an initial safety check performed with the bed rotated in all necessary planes—most importantly in Trendelenburg and left-sided tilt position. See Table 108-1 for a list of instruments typically required for minimally invasive colectomy (both laparoscopic and robot-assisted). In general, it is best for the surgeon to think of the target anatomy and how to best place the ports of access in a triangulated fashion to optimize dissection of the tumor, the relevant vasculature, and the accompanying lymph node basin. New thinking in robot-assisted surgery suggests putting the ports in a straight line opposite the target anatomy, but this is a surgeon-specific preference that is still in evolution.
Typical Instruments Required for Minimally Invasive Colectomy
| Laparoscopic | Robotic |
|---|---|
| Veress Needle (depending on the method used to create the pneumoperitoneum) | Same |
| Trocars: size 5 mm × 2, 12 mm × 2 | Varies |
| Camera: Endoscope 5 mm and 10 mm (our preference is a 30° or flexible tip camera) | Robotic camera |
| Laparoscopic bowel graspers | Robotic equivalent |
| Laparoscopic scissors | Robotic equivalent |
| Laparoscopic needle holder | Robotic equivalent |
| Laparoscopic bipolar vessel sealer device | Robotic equivalent |
| Small wound protector | Same |
| Laparoscopic linear stapler | Robotic equivalent |
| Circular stapler for end-to-end anastomosis (sizes 28 mm and 31 mm) | Same |
The right colon receives its blood supply from the superior mesenteric artery (SMA) through the ileocolic artery, the right colic artery, and the right branch of the middle colic artery. The transverse colon receives arterial blood from the middle colic artery, which arises from the SMA. The corresponding veins drain into the superior mesenteric vein (SMV). The left colon receives its blood supply from the SMA through the left branch of the middle colic artery, and from the inferior mesenteric artery (IMA) through the left colic artery and sigmoidal branches. The venous drainage is via the inferior mesenteric vein (IMV). The marginal artery of Drummond should be noted as it connects the IMA to the SMA and thus plays a central role in maintenance of perfusion to colonic segments even after intervening major vessels are divided. It is a continuous arterial circle or arcade along the border of the colon formed by anastomotic connections of the terminal branches of the SMA and IMA. This is important as it provides collateral flow when major vessels are occluded or stenotic.
Notably the junction of the SMA and IMA territories is found at the splenic flexure and the connection via the marginal artery is weak or absent and thus this area is known as a “watershed” area prone to ischemia or infarction. Thus, new bowel anastomoses are avoided in this area. The venous drainage follows the course of the arteries, except in the case of the IMV, which has a longer course than its homonymous artery. The IMV forms from the junction of the superior rectal vein and the sigmoidal branches and drains into the splenic vein, behind the pancreas. From its origin it joins the left colic vein and runs parallel to the left colic artery. From that point, until the junction with the splenic vein, the IMV travels without an accompanying artery.
The mesentery of the right colon overlies the right ureter, gonadal vessels, and Gerota’s fascia in addition to the SMA/SMV. The hepatic flexure is intimate with the liver and is anchored to the retroperitoneum by the hepaticocolic ligament and then lateral attachments to the abdominal wall are present down to the cecum along the White line of Toldt. The mesentery of the left colon overlies the iliac vessels, the left ureter, the left gonadal vessels, Gerota’s fascia covering the left kidney, and the left side of the pancreas, all covered by Toldt’s fascia. The mesentery of the left side of the transverse colon is attached to the inferior border of the pancreas. The splenic flexure of the colon is anatomically related to the lower pole of the spleen, and is anchored to the retroperitoneal structures by the phrenicocolic ligament. Preoperative CT scan imaging should be reviewed before the operation to identify all of the important anatomical structures mentioned above. Review of the CT scan also helps in planning the surgical approach and intraoperative dissection.
A segmental colectomy may be offered for resection of an endoscopically unresectable polyp, a high-risk malignant polyp (as above), or a biopsy-proven colon adenocarcinoma. Preoperative staging should occur with the imaging of the abdomen and chest to ensure no metastatic disease and the patients should be deemed medically fit to undergo the operation. Contraindications for segmental colectomy are the following: patients with locally advanced tumors requiring resection of adjacent organs, patients with peritoneal carcinomatosis, or patients with extensive peritoneal adhesions.
The laparoscopic approach to colon resection for cancer has several advantages over the open approach, such as shorter hospital stay, reduced postoperative ileus, earlier resumption of oral nutritional intake, reduced pain, and improved cosmesis. Despite initial reluctance to perform laparoscopic colectomy for colon cancer, due to concerns over port site recurrence and substandard oncologic outcomes, multiple studies have established the oncologic equivalence of laparoscopy with open surgery2,38–40. In the United States, recent work demonstrates that utilization of laparoscopic resection varies (0% to 67%) based on hospital referral region but that 32.5% of 93,786 colon resections were completed laparoscopically in spite of availability of equipment.41 There are no absolute contraindications for laparoscopic colon cancer surgery; however, patients with intestinal obstruction, patients with previous multiple abdominal surgeries and extensive abdominal adhesions, and those who cannot tolerate lengthy pneumoperitoneum may be better served with an open surgical approach. In this chapter, we review the indications for and essential steps involved in optimal colectomy for colon cancer describing laparoscopic, robot-assisted, and open approaches. For a more detailed step-by-step approach, the reader should seek other sources to supplement this work (e.g., totally robotic low anterior resection of the rectum42).
Dennis Fowler43 reported the first two laparoscopic sigmoid resections in 1991. That same year Jacobs44 reported his experience with 20 laparoscopic colectomies, primarily for treatment of benign conditions. Monson and colleagues45 were the first to report on a larger series of colon cancer patients treated laparoscopically. They described successful completion of laparoscopy in 33 of 40 patients, and a median hospital stay of 8 days. Since then, laparoscopic colectomy has become more widely accepted for treatment of malignant as well as benign disease. We will describe colectomies below in order from the perspective of open colectomy to minimally invasive colectomy.
For patients where extensive adhesions might be expected or if operative time must be minimized based on the patients co-morbidities, an open operation may be necessary. The positioning is supine and the preoperative preparation is the same as above. The arms can be placed out but the patient is secured to the operating room table in a similar fashion. A headlight worn by the surgeon or the assistant is essential. A midline incision is typically used; however, a transverse incision could be utilized if necessary. Once incision is made, a self-retaining retractor is placed and the abdomen is thoroughly explored. The placement of surgeon and assistant is the same as per the laparoscopic approach. The cecum and terminal ileum are retracted anteromedially by the right hand of the surgeon to expose the lateral and medial peritoneal attachments. Retract the colon medially and divide the lateral peritoneal attachments of the cecum and ascending colon along the white line of Toldt. The peritoneum and the colon are gently separated from the loose areolar tissue by finger dissection. Electrocautery is used to incise the peritoneum, and the left index finger of the surgeon then accesses this plane and can move toward the hepatic flexure as the assistant incises the exposed peritoneum allowing the terminal ileum, cecum, and distal ascending colon to be mobilized. It is important to keep the underlying kidney, right ureter, and gonadal vessels posteriorly in the retroperitoneum to avoid injury. Application of gentle anterior retraction on the mobilized right colon allows exposure of the duodenum and this is dropped unharmed away from the field with careful dissection away from the retroperitoneum. Small vessels at the level of the hepatic flexure near the base of the mesentery at the duodenum are fragile and may require suture ligation or clips before an avulsion occurs and distorts the planes of dissection. Divide the right renocolic ligament using electrocautery. Dissect distally along the colon until the gastrocolic ligament is encountered. Divide the gastrocolic ligament and complete mobilization of the hepatic flexure. Any adhesions to the gallbladder are taken down and the omentum is divided over the transverse colon with use of a sealing device or between clamps. The hepatic flexure is completely freed of omental or additional lateral attachments toward Toldt’s fascia. Care is taken to preserve the gastroepiploic vessels along the greater curve of the stomach. Release the terminal ileum by dividing the fold of Treves on its antimesenteric border. Ligate the lymphovascular pedicle. Identify the lymphovascular pedicles by retracting the small bowel to the left side of the abdominal cavity and elevating the right colon to expose the root of the mesentery. The ileocolic vessels are located at the caudal portion of the root of the mesentery. The right colic vessels are variable; they are predominantly a branch of the ileocolic (IC) but in rare circumstances are located at the middle of the root of the mesentery. The middle colic vessels are seen anteriorly to the area where the duodenum crosses below the SMA/SMV. Identify the SMA to prevent injury or inadvertent ligation. The ileocolic vessel is taken with suture ligation as is the right branch of the middle colic artery. We observe the principles of CME. The anastomosis is performed as described above for extracorporeal anastomoses with GIA-80 staplers and a TA-90 stapler. The abdomen is inspected after the anastomosis is placed back into the abdomen and proper orientation of the mesentery and bowel is confirmed. The fascia is closed and the skin approximated per the surgeon’s preference.
The indications for an extended right colectomy include a cancer located at the hepatic flexure to the mid-transverse or distal transverse colon, synchronous ascending and transverse colon cancers, and multiple adenomas, which may or may not be part of a genetic syndrome. A tumor that is located at or just distal to the hepatic flexure can be removed by extending the classic right hemicolectomy so that it includes ligation of the right branch of middle colic artery, as long as that gives a 5 cm free margin. Extended right hemicolectomy starts out exactly like a standard right hemicolectomy. The only exception is that the resection is extended to include ligation of the right branch or the entire trunk of middle colic artery based on the tumor location. Perform the Ileocolonic anastomosis as above. An extended right hemicolectomy, with ligation of the trunk of the middle colic artery and an anastomosis between the ileum and descending colon should be used for colon cancer arising between the two flexures. An anastomosis should be generally avoided in watershed areas (such as mid-ascending colon or splenic flexure). It should be noted that an alternative option for proximal to mid-transverse colon cancers is a transverse colectomy. The caveat of this procedure is that both proximal and distal ends of the resected bowel need to be well mobilized and assessed carefully to ensure a tension-free and well-vascularized anastomosis; hence, the extended right colectomy may be preferred in these settings. General issues regarding preoperative preparation of patients, patient positioning, antibiotic prophylaxis, bowel preparation, DVT prophylaxis, incision, and other considerations prior to extended right colectomy are similar to that of right colectomy.
The patient is positioned supine on the operating room table with both arms tucked, padded, and protected and properly strapped for security. In general, the surgeon is positioned opposite the pathology and direction of dissection, with the first assistant directly opposite; however, in this case both surgeons usually stand on the left side. In this case, the surgeon is on the left and may go between the legs if necessary with two monitors placed at the head of the table. Access to the abdomen can be attained via Veress needle or a cut-down technique utilizing a Hasson trocar. For a Veress needle, we create a small stab incision with a 15 blade in Palmer’s point (below the left costal margin in the mid-clavicular line (MCL)). With careful insertion of the Veress needle, it is possible to feel the rectus sheaths and the peritoneum as the needle penetrates through the different layers of the abdominal wall. We then place a 10 to 12 mm trocar in the supraumbilical region at the site of planned incision for extraction of the right colon. A laparoscope of choice is passed here and then two 10 to 12 mm trocars are placed in the left upper and left lower quadrants of the abdomen (lateral to the rectus musculature, see Fig. 108-4). Additional 5 mm trocars may be placed to assist in retraction (one possible option is shown in Fig. 108-4). The patient is put into Trendelenburg position and the left side of the table is turned down. If not performed previously, the omentum is retracted cephalad, and the small bowel to the patient’s left, in order to expose the colonic mesentery and the origin of the ileocolic vessels. In general, we use medial-to-lateral approach, although a lateral-to-medial approach has been described. The terminal ileum is located in addition to the base of the cecum and a medial-to-lateral dissection is begun and carried out until the ureter, kidney, and duodenum are safely out of the surgical field. The ileocolic vessels are put on tension and the peritoneum is scored medial to the vessels and dissection is carried out above the retroperitoneum making a “cave” while lifting the mesentery to maintain the correct plane and avoiding injury to the duodenum, ureter, or kidney. The ileocolic pedicle is taken with a vascular stapler or hemo-clips at its origin after proper exposure. Attention is then turned to the mobilization of the hepatic flexure. The transverse colon is grasped and the greater omentum is divided distal to the gastroepiploic vessels to the level of the middle colic artery. At this point the right branch of the middle colic artery can be taken at its origin with a vascular stapler or a vessel-sealing device based on surgeon preference. The omentum is further separated from the transverse colon and the hepaticocolic ligament is divided to complete the hepatic flexure dissection. This dissection is carried to the white line of Toldt to completely free the lateral attachments of the colon. A medial approach is then taken to ensure the terminal ileum and base of the cecum are freely mobile. A Babcock clamp is then placed on the cecum and positioned toward the extraction site. An adequate incision is then made at the supra-umbilical site to deliver the terminal ileum, cecum, ascending and transverse colon. We use a medium-size wound protector in this incision to minimize contamination and ease the evisceration of the specimen and surrounding bowel. Typically, we do an extracorporeal stapled side-to-side, functional end-to-end anastomosis (GIA-80 and TA-90) in continuity, ensuring proper lie of the mesentery and anti-mesenteric orientation. An intracorporeal anastomosis can also be completed laparoscopically using a 12 mm port for stapler introduction in the left lower quadrant in the MCL. Once the anastomosis is completed, the colon is returned to the abdominal cavity. The pneumoperitoneum is re-established by closing the wound protector with clamps. A final inspection of the abdomen is completed and the other trocars are removed under direct visualization. The wounds are closed per surgeon preference. In our practice, the fascia from the specimen retrieval site is irrigated and interrupted sutures are used to close the fascia with reabsorbable monofilament sutures. We prefer to close the skin of the midline incision with vertical mattress nylon stitches and the port sites with subcuticular monofilament reabsorbable sutures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree