Fig. 4.1
Surgical resection of RHCC in Eastern Hepatobiliary Surgery Hospital
4.2 Clonal Origin of Recurrent Hepatocellular Carcinoma
4.2.1 Monoclonal and Polyclonal Origin
The origin of RHCC has long been a major concern and discussion subject since at least 20 years ago [2]. Two major origin patterns of RHCC are concerned. One is intrahepatic metastasis (IM) origin, derived from intrahepatic micrometastases which cannot be recognized with the naked eyes and excised entirely during surgery due to the microvascular invasion (MVI) [8–10], and the residual cancer cells will proliferate post-surgery of PHCC. Obviously, IM pattern has the same clonal origin with the primary tumor and is also termed as monoclonal or single center origin. The other is multicentric occurrence (MO) origin, derived from cancer-adjacent hepatocytes, or de novo tumor clone, which undergo long-term genomic variation leading to carcinogenesis, due to persistent HBV/HCV infection in the patients of chronic hepatitis or liver cirrhosis [11–13].
4.2.1.1 IM-RHCC
Monoclonal origin hypothesis of tumor was proposed in the 1970s that tumors are derived from accumulation of mutation and clonal proliferation of single cells in the tumor cell population. RHCC is traditionally considered as monoclonal in origin, arising from intrahepatic metastasis or recurrence of the residual cancer cells post PHCC surgery, which prompted the initiation of clinical course into the late stage of invasion and metastasis.
As we know, an adult liver contains about 500 thousand–1 million hepatic lobules, mainly composed of liver cell plates and hepatic sinusoid, and each lobule is surrounded by 3–4 portal areas, containing vasculature of interlobular artery, interlobular vein, and interlobular bile ducts, which facilitates the MVI and intrahepatic metastasis of HCC and is the histological basis for IM-RHCC. The reported incidence of MVI in PHCC is 15% to more than 60% [8–10], and the larger the tumor is, the higher risk of MVI is, which is the reason that more than 80% of the RHCC are mainly within the liver. According to our statistics of a group of HCC cases, the MVI rate in small HCC (≤3 cm in diameter) was 6.9%, among which even a microcarcinoma (0.6 cm in diameter) has also been observed with MVI [14]. Moreover, MVI has been recognized as one of the most important pathological indicators of both postoperative recurrence and metastasis risk and clinical prognosis after surgery [15].
4.2.1.2 MO-RHCC
The monoclonal theory of tumor proposed in 1976, that monoclonal proliferation is one of the main features of the tumor, laid an important theoretical foundation for the differentiation of tumor lesions and proliferative lesions, supported by most researchers considering multiple nodules, recurrent lesions, satellite lesions, and extrahepatic metastasis as monoclonal in origin. Until the late 1980s, with the development of molecular biological techniques which promoted the research of clonal origin of HCC, MO-RHCC was then discovered.
More attention has been paid to the mechanism of polyclonal origin of RHCC since the late 1990s. One of the reasons is the fact that more than 80% of PHCC patients in China have HBV-related chronic hepatitis or liver cirrhosis. HBV-DNA integrate into the genome of the host liver cell randomly, and precancerous lesions such as atypical hyperplasia and dysplastic nodules distribute throughout the liver with heterochronous carcinogenesis and multicenter origins. These contribute to the pathological basis of MO-RHCC. According to current RHCC molecular detection, the proportion of MO-RHCC is about 15–30%.
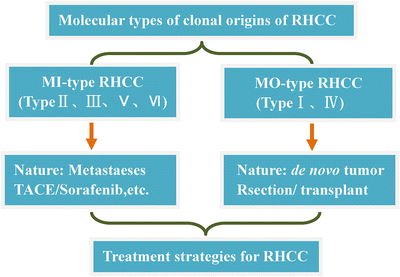
The interval between PHCC excision and RHCC occurrence is variable from less than 1 year to more than 10 years, making it difficult to preserve paired fresh tissue specimens for a long period to detect molecular clones. Supported by the National Natural Science Foundation of China, the author explored the microsatellite LOH pattern difference detection and determined the six molecular clone types or origin patterns of RHCC, via screening loss of heterozygosity (LOH) of high-frequency microsatellite DNA: type I, single nodular polyclonal RHCC via de novo tumor clone; type II, single nodular monoclonal RHCC via intrahepatic metastasis of PHCC; type III, single nodular monoclonal RHCC with its intrahepatic metastasis nodules; type IV, polyclonal and multinodular MO-RHCC; type V, single nodular polyclonal RHCC with its intrahepatic metastasis nodules; and type VI, combined polyclonal MO-RHCC and metastatic nodules from PHCC [16].
Differences of the above six types reflect the different mechanisms and pathways of RHCC providing a reference for clinical treatment of RHCC patients individually based on the clonal characteristics of HCC. As reported in the current literature, MO-RHCC and IM-RHCC constituted 15–30% and 70–85% of total RHCC, respectively, and the patients’ average survival times post-surgery were 130 months and 80 months (P < 0.05), respectively, suggesting the better curative effect of reoperation for MO-RHCC [17].
4.2.2 Multifocal Growth and Multicentric Origin
Field cancerization theory was proposed by Slaughter in 1953 and remains to constitute the theoretical basis of pathogenesis of epithelial tumor. He hypothesized that one or multiple precancerous epithelial cells underwent sequential tumor genetic or epigenetic transformation to form primary field tumor (PFT) due to the impact of carcinogenic factors, whose persistent existence would facilitate the same genetic mutation and the formation of second field tumor (SFT) derived from the precancerous epithelial cells around the PFT [18, 19]. Theoretically, the molecular range of precancerous lesions is larger than the actual range of solid tumors. And dynamic multistage evolution and clonal selection of precancerous lesions in the region would lead to multifocal tumors with or without heterochrony. Different from tumors with multicentric origins, if PFT, SFT, and local recurrence (LR) of PFT share the same molecular variation or genetic alterations, they are determined as the same or monoclonal origin. However, if a tumor derives from another region, it’s determined as a second primary tumor (SPT) and is a multicentric origin tumor. Thus, not all multifocal or recurrent tumors are multicentric origin tumors arising from de novo tumor clone, and it is apparently difficult to differentiate the clonal origins of PET, SFT, LR, and SPT clinically or histologically despite their different pathogenesis.
Slaughter’s field cancerization hypothesis has already been confirmed in a variety of common epithelial tumors and is a practical reference for the investigation of clonal origin of RHCC. Studies have shown the significant difference among genetic methylation frequencies of HCC tissues, surgical margin, chronic hepatitis, and liver cirrhosis, suggesting the regional canceration in the liver, which reveals the variation and complexity of pathogenesis and origins of RHCC. Previous discussions of RHCC diagnosis, prevention, and treatment are more related to IM-RHCC, while we now should take the prevention and treatment of MO-RHCC into account, including the determination of molecules of PHCC, the prevention and repairing of genetic mutations and the progression of precancerous lesions in HBV/HCV infection areas, and early identification of precancerous cells with normal morphology and highly malignant tendency in the field. So, local canceration hypothesis is of practical significance to guide surgical resection, prevention, diagnosis, and treatment of PHCC. It can be deduced from the hypothesis that hepatic cells around HCC(T) have already had the genetic mutations to varying degrees and are in different stages of canceration in the patients with HBV/HCV infection, while their morphology remains basically normal. And these precancerous cells will continue the process of carcinogenesis to form LR or SPT after the so-called radical excision. Therefore, only when the PFT and all the cells with cancerous genetic mutations and biochemical alterations are excised will it be possible to completely prevent the recurrence of all forms of monoclonal tumor relapses or new tumor rerecurrences theoretically (Fig. 4.2).
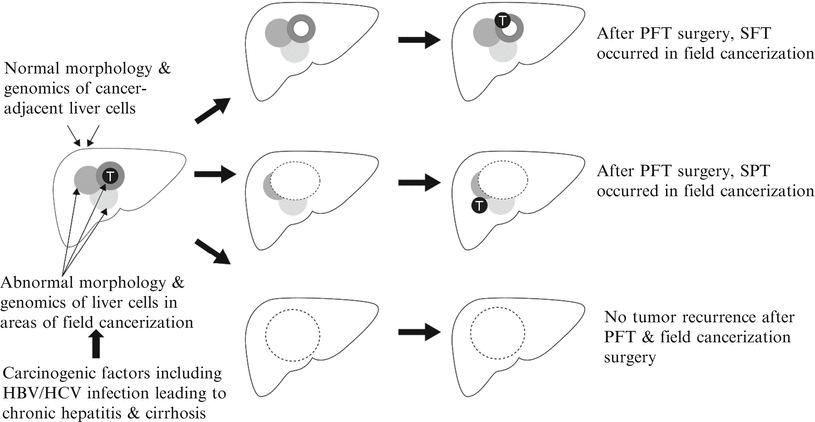

Fig. 4.2
Schematic diagram of the relationship between HCC and regional carcinogenesis and treatment mode
Moreover, the clonal origin of liver tumors may involve malignant transformation and clonal selection within different tissues in terms of hepatic progenitor cells (HPCs). As mentioned above, determination of the clonal nature of tumors should be based on molecular pathology due to the complex and diverse clone types of RHCC. Microsatellite loss of heterozygosity (LOH) was applied for clone identification to diagnose the first case of multi-origin primary malignant tumors of the liver, which received the first HCC resection and later resection of intrahepatic cholangiocarcinoma and fibrosarcoma [20]. To improve molecular pathodiagnosis in the deparment of pathology, great importance should be attached to set up tumor molecular cloning detection methods [21].
4.3 Clinical Features of Recurrent Hepatocellular Carcinoma
4.3.1 Pathological Diagnostic Criteria
Shimada et al. described the characteristics of multiple nodular intrahepatic metastasis (IM) of HCC grossly and microscopically including ① obvious derivation from portal vein tumor thrombus, ② multiple satellite nodules around the primary tumor, and ③ histological similarities between solitary tumors adjacent to the primary tumor [22]. As to RHCC, IM can be diagnosed if the recurrent tumor has a moderate to low degree of differentiation and a similar or lower grade to the original tumor. Besides, the Liver Cancer Study Group of Japan put forward the histological diagnostic criteria of MO including [23] ① moderately to poorly differentiated primary tumor and highly differentiated recurrent tumor, ② highly differentiated PHCC and RHCC, ③ RHCC with precancerous lesions or highly differentiated HCC surrounding poorly differentiated HCC or nodule-in-nodule appearance, and ④ RHCC with higher differentiation than that of PHCC. And the diagnostic criterion for IM is RHCC with poorer differentiation than that of PHCC. However, these histological criteria can’t be applied to diagnose most RHCC, due to the extremely small proportion of highly differentiated HCC and high-grade dysplastic nodules (HGDN) in clinical practice. Moreover, the determination of differentiation degree and precancerous lesions is not completely object, easily affected by subjective factors such as the working experience of the pathologists. Thus it is inaccurate to determine the clonal origin of RHCC simply based on the morphohistology of the tumors.
4.3.2 Clinical Diagnostic Criteria
Differentiation between recurrence of a residual lesion and a de novo tumor post-surgery is a key to develop therapeutic strategy and predict clinical prognosis scientifically. However, due to the inaccuracy of determining the clonal origin of a RHCC according to clinical manifestations, doctor’s experience plays a major role. Currently, single center recurrence (IM-RHCC) refers to recurrence within 2 years post-surgery (short-term recurrence), and multicentric recurrence (MO-RHCC) refers to recurrence more than 2 years after tumor excision (long-term recurrence). Li et al. [24] detected the pattern of P53 mutation via PCR-SSCP and divided 12 cases into two groups, single center recurrence (6.5 ± 3.25 months) and multicenter recurrence (33.8 ± 17.8 months). They concluded that recurrence within 2 years post-surgery derived from single center or multicenters, while recurrence more than 2 years after surgery mainly derived from multicenters and thus was secondly primary carcinoma. However, our previous researches on molecular clone detection showed there was overlap of recurrence interval between IM-RHCC and MO-RHCC. For instance, in a molecular clone detection, monoclonal origin or recurrence of residual lesions was found in a RHCC 8 years post-surgery [4]. The author also reported a patient who occurred hepatic and colon metastasis from breast cancer 13 years after the surgery of breast cancer, and the patient received a second surgery for the metastatic tumors [25], indicating that residual cancer cells could stay silent or in a tumor-dormant state in vivo for a long time and proliferate again due to certain microenvironment changes even to metastasize.
The difference of serum AFP levels between PHCC and paired RHCC patients was also found to be associated with the difference of clonal origins. The recurrence intervals in group A (significantly different serum AFP levels between PHCC and RHCC patients) and group B (similar serum AFP levels between PHCC and RHCC patients) were 34.1 ± 3.8 months and 24.6 ± 2.7 months, respectively (P < 0.05), and recurrence intervals in group A type II and group B type II (recurrent tumors of different liver lobes) were 39.4 ± 5.9 months and 21.3 ± 4.1 months (P < 0.05), suggesting RHCC in group A has the features of MO-RHCC, such as the relatively longer growth periods of neoplasms in multistage growth pattern, and the difference of serum AFP levels may also reflect differences in tumor cell clone characteristics. Huang et al. [26] divided 82 RHCC into IM type and MO type post-surgery according to histological criteria; the recurrence intervals were 10.78 ± 7.9 months and 47 ± 31.69 months (P < 0.001), suggesting a possible relationship between recurrence intervals and tumor clonal origins. In principle, IM-RHCC-derived residual tumor post the first HCC excision is usually complicated with MVI or satellite foci formation, and this should primarily consider multimodality therapy including interventional therapy (such as radiofrequency ablation, hepatic artery embolization chemotherapy, and biological therapy), while MO-RHCC is a de novo tumor in nature and is more suitable to be excised or treated by liver transplantation, the same effect of which can be acquired with the first surgery of PHCC. On this basis, the author proposed the relevance between clonal origin types and individualized treatment modes of RHCC (Fig. 4.3).