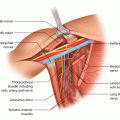
Fig. 14.1
Breast cancer presented with visible skin retraction
Table 14.1
The level of suspicion for malignancy on clinical examination scale
P1 | Normal |
|---|---|
P2 | Benign |
P3 | Uncertain |
P4 | Suspicious |
P5 | Malignant |
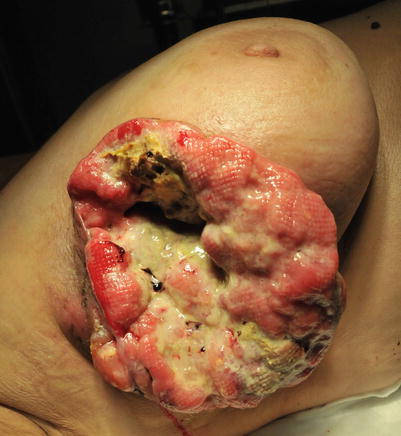
Fig. 14.2
Locally advanced breast cancer with necrosis, bleeding and secondary bacterial infection
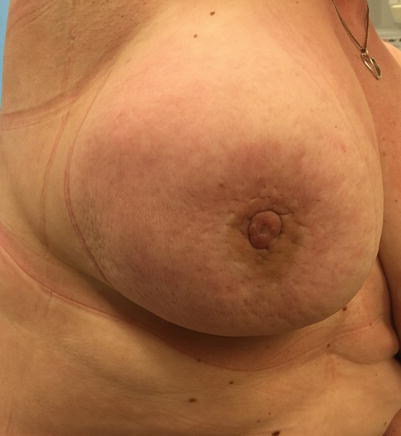
Fig. 14.3
Inflammatory breast cancer; note redness and oedema of the skin (peau d’orange) and nipple retraction
Whenever the above-mentioned manifestations are noted, the corresponding diagnostics should be performed prior to further treatment [37].
14.3 Diagnosis of Breast Cancer
The ultimate diagnosis of breast cancer is histopathological, but the diagnostic process and treatment planning require that all patients undergo triple assessment. This consists of clinical examination, imaging investigations and a biopsy. The EUSOMA quality indicators specify that physical examination and imaging of the breast should be performed in at least 90% of breast cancer patients and definitive diagnosis made prior to first treatment in at least 80% of patients [38]. In palpable breast cancer, triple assessment should be performed in 95% of patients [5]. These steps are described in detail below:
- 1.
Clinical Examination
All women should have a detailed medical history and physical examination performed.
- 1.
Medical History
A detailed history of previous and current diseases, surgical or other interventions and medications should be recorded. A family history of cancer should be recorded to assess the risk of a familial tendency which may affect risk and management. A systematic gynaecological history should be recorded to include the age at menarche, a number of completed pregnancies and the age at first childbirth and history of breast-feeding. In premenopausal women, the date of the last period and the use and duration of hormonal contraception should be recorded. In postmenopausal women the age at menopause, the cause of menopause and the use and duration of hormonal replacement therapy should be recorded. Of particular interest is a history of previous breast complaints or surgeries, especially previous breast biopsies or breast augmentation or reduction.
- 2.
History of the Presenting Complaint
The duration of symptoms and whether they are stable, resolving or worsening and whether the symptoms are cyclical or not. The latter is more in keeping with a benign diagnosis.
- 3.
Physical Examination
Physical examination should include examination of the breasts, chest region and the regional lymph node basins in the axilla and infra- and supraclavicular fossae.
Inspection
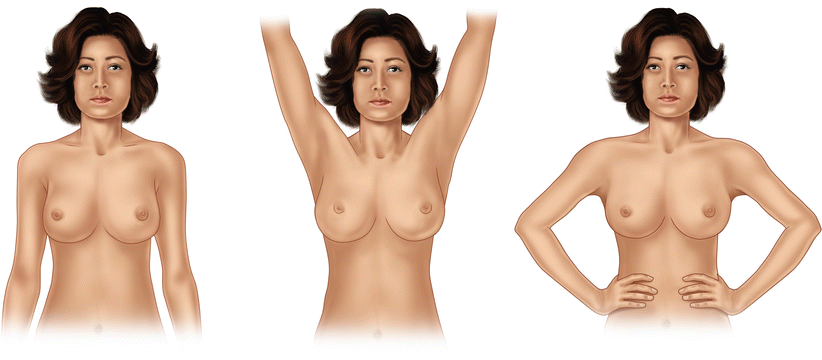
A chaperone should always be present. Inspection starts in the upright position with the patient unclothed to the waist. The patient is asked to elevate her arms above her head and finally to put them onto the hips and contract the pectoral muscles. During all three phases of inspection, the clinician should observe for breast asymmetry (size, shape or distortion), skin changes (skin retraction, erythema, ulceration, oedema or eczematous changes) and changes to the nipple (symmetry, retraction, inversion, nipple discharge or crusting). In cases of nipple discharge, it is very important to record whether the discharge is unilateral or bilateral, uniduct or multiduct and spontaneous or induced and the colour of the discharge (especially if blood stained) (◘ Fig. 14.4).
Palpation
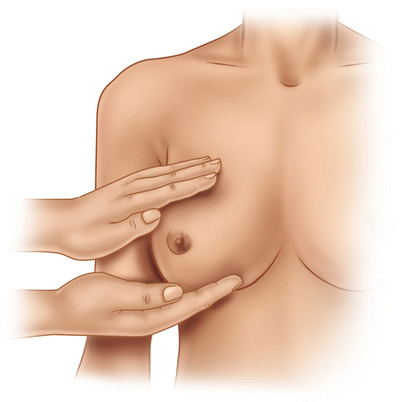
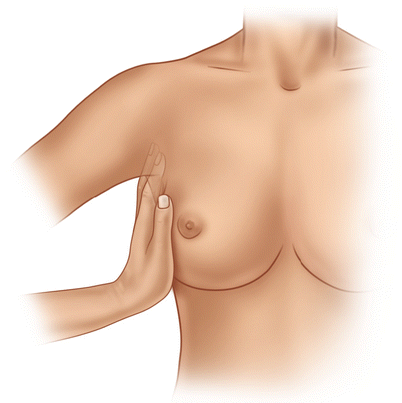
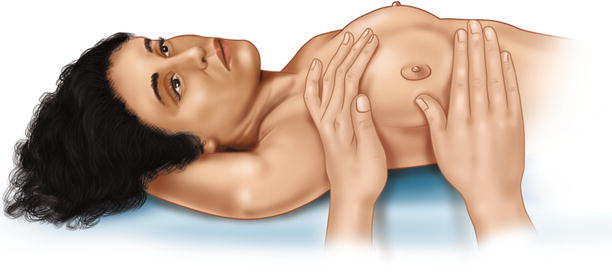
Inspection is followed by thorough palpation of the breast, axilla and lymph nodes in the supra- and infraclavicular fossae (◘ Figs. 14.5, 14.6, and 14.7). If a lump is found, its size, mobility, position (in the breast and relative to the nipple) and consistency should be assessed. If the lump is fixed, it should be noted whether to the chest wall, to the skin or to both.
- 4.
Imaging of Early Breast Cancer
Clinical examination is followed by imaging investigations of the breasts: mammography, ultrasound and in some cases MRI. The goal is to further characterise breast lesions and to assess the number (multifocality, two or more lesions in the same quadrant or separated by less than 4 cm, or multicentricity, two or more lesions in different quadrants or separated by more than 4 cm) and bilaterality of suspicious lesions. All these data are of paramount importance for determining whether breast-conserving surgery is possible. These imaging investigations are described in details in ► Chap. 12, including the BIRADS classification system. This chapter will focus on the use of these methods during the diagnostic workup of a clinically suspicious breast lesion.
Mammography
Diagnostic bilateral two-view mammography is usually performed in any woman over the age of 35. The only two exceptions are pregnancy and young age (see below).The standard views are the craniocaudal (CC) and mediolateral oblique (MLO); however, additional mammography techniques may be indicated to further evaluate the breast lesion, like magnification views of suspicious areas with focal compression. Whenever possible it should be performed before needle biopsies as they may produce changes (i.e. hematoma) which may reduce the diagnostic value of the mammogram. It is also advisable to obtain any previous mammograms of the patient for comparison. Diagnostic mammography is known to be more sensitive but less specific compared to screening mammography due to the likely more advanced stage of symptomatic disease [39]. Mammography sensitivity is diminished by high breast density, young age, hormone replacement therapy, some biological subtypes (i.e. invasive lobular carcinoma) and the presence of breast implants. If implants are present, special views may be taken to «see around» the implant, but these do not fully compensate for the loss of sensitivity in these women.
There are several mammographic abnormalities which are suspicious for breast cancer: masses, architectural distortions and microcalcification. Microcalcifications are particularly important in diagnosing ductal carcinoma in situ (DCIS) [40].
In young patients less than 30–35 years of age, ultrasound is the modality of choice for breast imaging [41], but US should ideally be used on older women as an adjunct to mammography and to undertake a guided biopsy (which has a lower false-negative rate than a clinically guided biopsy). In young women, high breast density limits the value of mammography. However, if breast cancer is subsequently diagnosed, then bilateral mammography should be performed as in older women.
Diagnostic evaluation of a newly discovered breast mass in a pregnant woman is challenging. Physiological changes during pregnancy make physical examination difficult due to increased density and nodularity, and for the same reasons mammographic sensitivity is low. The imaging method of choice is therefore ultrasound although mammography with proper shielding is safe for the foetus [42]. Any clinically or ultrasonically discrete or suspicious lesion should be subjected to core biopsy, and it is important to notify the pathologist of the pregnancy as otherwise the highly proliferative state of the pregnant breast epithelium might cause undue concern. If breast cancer is discovered, then mammography with foetal shielding may be performed.
Ultrasound
Ultrasound is an important diagnostic investigation to further characterise a clinically and/or mammographically suspicious breast lesion. It is also now routinely used to stage the regional lymph nodes (see below). It has a very high sensitivity and specificity in experienced hands [43]. There are several indications to perform a breast ultrasound:
To characterise a solid mass or architectural distortion present on mammogram. A benign US result should not deter biopsy of a lesion. In a Dutch study the added value of ultrasound to clinical examination and mammography was reported: the sensitivity, specificity and positive and negative predictive values for clinical examination plus mammography plus US were 96.9, 94.8, 39.2 and 99.9 per cent, while the corresponding values for clinical examination plus mammography were 91.5, 87, 19.7 and 99.7 per cent, respectively [44].
To further characterise a palpable lesion not clearly seen on mammogram. This is particularly the case when a lump is discovered by self-examination, and the breasts are dense on mammography giving the latter a lower sensitivity. In order to distinguish an area of low suspicion nodularity of the breast from a discrete breast mass, an ultrasound is of great value. The negative predictive value for malignancy after both a normal mammogram and ultrasound is 99. 8% [44, 45]. Therefore, a biopsy may be avoided in some cases, although all clinically discrete, asymmetric lesions should be biopsied in women over the age of 25.
To identify cystic lesion of the breast and to distinguish simple cysts from a complex cysts. Simple cysts can be aspirated or left untreated; however, a complex cyst may require a formal biopsy [46].
In young women age < 30, ultrasound is the primary imaging investigation [47]. In most cases an ultrasonically benign lesion is found and can be needle biopsied or simply followed up a few months later. In clinically and ultrasonically suspicious lesions, a needle biopsy and mammogram should be performed.
In pregnant and lactating women, ultrasound is the primary imaging modality, followed by mammography with shielding only in suspicious cases.
In patients with uniduct nipple discharge that is milky, serous, bloody or serosanguineous, an ultrasound examination may reveal a papillomatous lesion (or several papillomas) which may be biopsied under US guidance.
Breast inflammation/abscesses are another indication for ultrasound as it may facilitate drainage of pus, especially in complex multiloculated sepsis, or reveal an underlying malignancy in breast inflammation that does not respond to antibiotic treatment.
Second-look ultrasound after an MRI investigation of the breast. MRI has a low specificity and is not generally used to undertake an image-guided biopsy (unless special equipment is available). Ultrasound is a useful tool to reassess the MRI result and biopsy the lesion. However, only about half of MRI visible lesions are visible on ultrasound.
To search for multifocality/multicentricity or bilaterality in already proven breast cancer patients prior to treatment, particularly women with lobular cancer who have a preference for breast-conserving surgery. MRI would be the preferred modality in this setting if available however.
Ultrasound is an excellent tool to guide needle biopsies of suspicious lesions and to guide the marking of a tumour with a clip prior to neoadjuvant systemic therapies.
MRI
The use of MRI in the diagnostic evaluation of breast lesions has increased significantly in the last two decades. MRI is highly sensitive but less specific than other imaging methods [19]. Therefore, the indications for the use of MRI are in many scenarios still a matter of controversy as it may delay the diagnostic process, lead to unnecessary biopsies and result in overtreatment (elevated ipsilateral and bilateral mastectomy rates) without any proof of survival benefit for the patients. Thus, MRI should not be routinely used in every breast cancer patient. When used, the MRI finding alone without biopsy of the suspicious lesions should not be used to divert patients from breast-conserving surgery to mastectomy.
There are several clinical situations where MRI is of undisputed value in breast lesion diagnostics. The use of MRI as a screening method in high-risk women is discussed elsewhere in ► Chap. 6.
In patients with an equivocal or discordant physical examination, mammography and ultrasound results to enhance the diagnosis and staging of breast cancer.
In occult breast cancer with axillary adenopathy or Paget’s disease of the nipple with no mammographic, sonographic or physical evidence of the primary cancer.
For patient with planned neoadjuvant systemic treatment to assess the initial extent of the disease and to follow up the effect of the systemic therapy. Interpretation of the effect of treatment should be done with caution as MRI can overestimate the extend of the disease after neoadjuvant systemic treatment.
Patients with invasive lobular cancer.
Patients with breast implants.
For patients with chest wall involvement to accurately determine the feasibility of surgery.
In high-risk women where the probability of contralateral breast cancer is elevated (i.e. family history, prior radiotherapy to the chest wall, e.g. mantle radiotherapy for Hodgkin’s lymphoma).
- 5.
Biopsies
Percutaneous biopsy of every suspicious palpable lesion or imaging identified breast abnormality is obligatory European quality indicators mandate at least 80% (desirable target 90%) of women with breast cancer (invasive or in situ) should have a preoperative definitive diagnosis of breast cancer [38]. Diagnostic surgical biopsy should only be performed when percutaneous biopsy is not technically feasible.
There are several methods of breast biopsy in use today: fine needle aspiration (FNA) biopsy, core biopsy (CB), vacuum-assisted biopsy, punch biopsy and incisional and excisional biopsy. They can be performed free-hand or image-guided, depending on the clinical situation. In all cases, the biopsy result should be compared with the imaging results to ensure concordance at the MDT. In all cases with discordant results, further diagnostics are indicated. All these methods are briefly reviewed below:
FNA
Despite the steady decline in the use of the FNA in the diagnosis of a breast lesion, it is still a valuable method in experienced hands and some clinical situation. It also allows hormonal receptor status and Her-2 status to be evaluated in some cases, although less reliably than core biopsy. The advantages of FNA are as follows: it is quick to perform, requires less patient preparation, provides rapid results and is low cost. The disadvantages are as follows: it provides less material for diagnosis and lower and variable sensitivity and specificity, ranging from 65–98% to 34–100%, respectively [48]. It also does not give full histological information (invasiveness, grade, tumour architecture). Lastly, clip placement into the biopsy site is not possible. Nevertheless, it is very useful in palpable lesions, particularly clinically suspicious cysts; when a cyst is fully drained and the aspirate is non-blood stained, it can be discarded. The FNA is also an alternative approach in patients taking antithrombotic treatments as there is less risk of bleeding. Because of the lower sensitivity of FNA, when its results are inconclusive, CB should be performed. In non-palpable lesions FNA is not an appropriate biopsy method as it is – for various reasons – non-diagnostic in a much higher proportion of patients [49].
US-guided FNA is a popular method also to stage the axillary lymph nodes [50]. The indications to perform FNA of the lymph nodes are based on established US criteria [51]. In the setting of FNA of lymph nodes, it is more discriminant than FNA of the breast, as any epithelial cells are regarded as abnormal because a node should just contain lymphoid cells.
Core biopsy
As mentioned above, CB is the gold standard of percutaneous biopsy techniques. In contrast to FNA it has higher sensitivity and specificity, it allows full histopathological diagnosis of the lesion including all important biological features of the tumour and it allows positioning of clips into the biopsy site which is extremely important when further surgery is needed and/or when neoadjuvant systemic therapies are planned [49]. It is more time-consuming and painful and is performed under local anaesthesia. It requires a small skin incision, and patients on antithrombotic treatments should have these stopped to allow clotting to nearly normalise before biopsy. The final diagnosis takes longer and it is more costly. Despite these disadvantages CB is the only appropriate method in non-palpable lesions. A variant of the CB is the vacuum-assisted core biopsy (VACB) which allows harvesting of more material for diagnostic tests. It is most often used in patients with microcalcification or where a previous CB has yielded inadequate or discordant results.
After VACB or core biopsy for microcalcification, the specimen should be radiographically checked for the presence of the biopsied lesion, thus confirming that a representative sample has been acquired.
In non-palpable lesions, an image-guided biopsy is performed, using the imaging method which allows visualisation of the lesion. However, many lesions can be visualised by several imaging techniques, and in this case the easiest one is chosen. For instance, when small mammographic stellate lesions are also clearly seen on ultrasound, most radiologist would perform CB under US guidance. Or when a second-look US reveals an MRI-detected lesion, US will be used to guide the biopsy. In cases of microcalcification however the usual way to guide the biopsy is by stereotactic (mammography) guidance. The most demanding way to guide the biopsy is by MRI guidance which is only performed when the lesion cannot be visualised by other methods. This is only available in certain specialised centres.
Punch biopsy
With a punch biopsy a sample of a skin lesion is obtained in order to differentiate between benign and malignant skin lesions such as Paget’s disease and skin recurrence.
Galactography and ductoscopy
In patients with nipple discharge, several diagnostic options are available. As cytological examination of nipple discharge is relatively insensitive for detecting an underlying malignancy and a negative result should not prevent surgical excision of the duct, galactography may be used to show filling defects in the ductal tree. In a large series the sensitivity for detecting breast cancer was 54% and the specificity 98%, respectively [52].
A recent meta-analysis showed that ductoscopy detects about 94% of all underlying malignancies in patients with pathological nipple discharge but does not permit reliable discrimination between malignant and benign findings [53]. It is not in widespread use clinically.
Surgical biopsy
Excision biopsy
As stated before, surgical excision biopsy should be performed only in selected situations.
First, when the percutaneous biopsy did not definitively diagnose the lesion, especially if VACB is not available.
Second, when the result of the percutaneous biopsy showed a lesion which might be associated with an underlying breast cancer so a larger volume sample is mandated (i.e. radial scar, papilloma, atypical ductal hyperplasia, lobular carcinoma in situ).
Third, when the biopsy result and the imaging features are discordant.
Finally, when a percutaneous biopsy is not technically feasible (i.e. the position of the lesion). Surgical biopsy is usually associated with general anaesthesia and is linked to more complications compared to percutaneous biopsies, like hematoma or infection. For non-palpable lesions, a variety of localisation methods are in use and are discussed in more detail in ► Chap. 18.
Incisional biopsy
Incisional biopsy is rarely performed, usually in cases when the excision of the entire lesion would compromise the aesthetic appearance of the breast, for example, in cases with extensive microcalcification or a large mass on which percutaneous biopsy has been non-diagnostic.
- 1.

Fig. 14.4
Patient and arm positions during inspection

Fig. 14.5
Breast palpation in the upright position of the patient

Fig. 14.6
Palpation of the axillary lymph nodes

Fig. 14.7
Breast palpation in supine position
14.4 Staging
Patients can be staged clinically or pathologically, the latter being considered more accurate. Clinical staging can be used initially to guide treatment and subsequently changed accordingly by histopathological results. Today the seventh version of the TNM system is used to stage patients by the T (tumour), N (nodes) and M (metastasis) characteristics of the disease [11]. Patients are further grouped into stages from Stage 0 to Stage IV. The TNM system is based on retrospective survival data (◘ Table 14.2).
Table 14.2
Tumour node metastases (TNM) staging system for carcinoma of the breast
Primary tumour (T) a, b, c | |||
TX | Primary tumour cannot be assessed | ||
T0 | No evidence of primary tumour | ||
Tis | Carcinoma in situ | ||
Tis (DCIS) | Ductal carcinoma in situ | ||
Tis (LCIS) | Lobular carcinoma in situ | ||
Tis (Paget’s) | Paget’s disease (Paget disease) of the nipple not associated with invasive carcinoma and/or carcinoma in situ (DCIS and/or LCIS) in the underlying breast parenchyma. Carcinomas in the breast parenchyma associated with Paget’s disease are categorised based on the size and characteristics of the parenchymal disease, although the presence of Paget’s disease should still be noted | ||
T1 | Tumour ≤20 mm in greatest dimension | ||
T1mi | Tumour ≤1 mm in greatest dimension | ||
T1a | Tumour >1 mm but ≤5 mm in greatest dimension | ||
T1b | Tumour >5 mm but ≤10 mm in greatest dimension | ||
T1c | Tumour >10 mm but ≤20 mm in greatest dimension | ||
T2 | Tumour >20 mm but ≤50 mm in greatest dimension | ||
T3 | Tumour >50 mm in greatest dimension | ||
T4d | Tumour of any size with direct extension to the chest wall and/or to the skin (ulceration or skin nodules) | ||
T4a | Extension to the chest wall, not including only pectoralis muscle adherence/invasion | ||
T4b | Ulceration and/or ipsilateral satellite nodules and/or oedema (including peau d’orange) of the skin, which do not meet the criteria for inflammatory carcinoma | ||
T4c | Both T4a and T4b | ||
T4d | Inflammatory carcinomae | ||
Posttreatment ypT. f The use of neoadjuvant therapy does not change the clinical (pretreatment) stage. Clinical (pretreatment) T will be defined by clinical and radiographic findings, while y pathologic (posttreatment) T will be determined by pathologic size and extension. The ypT will be measured as the largest single focus of invasive tumour, with the modifier «m» indicating multiple foci. The measurement of the largest tumour focus should not include areas of fibrosis within the tumour bed | |||
Regional lymph nodes (N) | |||
Clinical | |||
NX | Regional lymph nodes cannot be assessed (e.g. previously removed) | ||
N0 | No regional lymph node metastases | ||
N1 | Metastases to movable ipsilateral level I, II axillary lymph node(s) | ||
N2 | Metastases in ipsilateral level I, II axillary lymph nodes that are clinically fixed or matted; or in clinically detectedg ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastases | ||
N2a | Metastases in ipsilateral level I, II axillary lymph nodes fixed to one another (matted) or to other structures | ||
N2b | Metastases only in clinically detectedg ipsilateral internal mammary nodes and in the absence of clinically evident level I, II axillary lymph node metastases
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||