If a reliable measurement of ionized serum calcium (iCa) is available, this measurement may be useful especially in selected patients (with hyper- or hypoalbuminemia, thrombocytosis, Waldenström macroglobulinemia and myeloma); moreover, it optimizes diagnostic accuracy in patients with normal serum albumin concentrations and acid-base balance, whereas in patients affected by NCpHPT, its assay is mandatory [8]. Primary hyperparathyroidism is the most common cause of hypercalcemia, but when confirmed, all of its causes should be considered. If a long-standing asymptomatic hypercalcemia is confirmed by the review of previous serum calcium values the suspicion of pHPT is higher. The finding of elevated or inappropriately normal (in the higher half of the normal range) serum PTH with concomitant hypercalcemia usually allows the diagnosis of pHPT to be confirmed. PTH should be measured by immunometric assay of, at least, 2nd generation. It was recently recognized that these assays detect not only intact 1-84 PTH, but also large amino-truncated PTH fragments, of which the most abundant is PTH (7-84). Third generation immunoassays that detect exclusively the biologically active PTH 1-84 (whole PTH or bioactive PTH) by recognizing both the extreme amino-terminal and carboxy-terminal ends of the molecule have been proposed recently as the golden standard for diagnosis of pHPT, particularly in patients with impaired renal function. However, other subsequent studies have found that the newest assays do not warrant an increased sensitivity in the diagnosis of pHPT with the exclusion of patients with advanced renal failure. Thus, either intact PTH (second-generation PTH assay) or whole PTH (1-84) assays (third generation PTH assays) can be used for diagnosis of pHPT [8]. The serum phosphorus is reduced in approximately 25% of patients with pHPT; hence, the sensitivity of its measurement for the diagnosis of pHPT is low. In all patients with pHPT, 25-hydroxyvitamin D (25-OH-D) should be measured: 25-OH-D levels in the deficient range (i.e. <20 ng/mL) were found in 27–53% of these patients. Also 24-h urine calcium and phosphorus are generally increased in pHPT, though concomitant vitamin D deficiency may induce normal or reduced excretion of these minerals so that they do not represent specific clue of pHPT. Moreover, persistent hypocalciuria after adequate repletion of vitamin D may indicate the presence of familial hypocalciuric hypercalcemia (FHH). The measurement of calcium/creatinine clearance ratio (Ca/Cr CR) in a 24 h urine collection allows the diagnosis to be confirmed. The Ca/Cr CR less than 0.01, on a normal calcium diet distinguishes between pHPT and FHH but the diagnosis of FHH must be confirmed by CASR sequence testing in cases of doubt [8]. Though not useful in clinical practice, both bone formation (serum total and bone-specific alkaline phosphatases, osteocalcin, type-1 procollagen [C-terminal/N-terminal]) and resorption turnover markers (urinary total pyridinoline, urinary free deoxypyridinoline, urinary collagen type-1 cross-linked N-telopeptide, urinary or serum collagen type-1 cross-linked C-telopeptide) may be increased in pHPT.
6.7.1 Diagnosis of Normocalcemic Primary Hyperparathyroidism
The diagnosis of NCpHPT can be made in subjects in whom serum ionized calcium and total calcium corrected for serum albumin levels are persistently normal and the concomitantly PTH level is persistently elevated [8]. The diagnosis of NCpHPT can be confirmed when all causes of secondary hyperparathy-roidism are ruled out.
Among these, the more frequent is hypovitaminosis D: an adequate supplementation of cholecalciferol should be administered to raise 25-OH-D levels above 20 ng/mL (50 nmol/L), as the minimal required level, but 30 ng/mL (75 nmol/L) is desirable. In this way, after at least three months of supplementation, serum parathormone levels normalized if a secondary hyperparathyroidism was present, as well as a real NCpHPT, or, sometimes, a latent traditional hypercalcemic pHPT would manifest itself.
Another secondary cause of hyperparathyroidism that must be ruled out is chronic kidney disease: it seems reasonable to require the GFR to be greater than 60 cc/min if the diagnosis of NCpHPT is to be substantiated.
Other conditions that must be excluded are: drugs known to increase parathormone levels (like thiazides, bisphosphonate, denosumab, and lithium), hypercalciuria and gastrointestinal disorders associated with calcium malabsorption.
The diagnosis of NCpHPT must include normal serum total calcium and ionized calcium on several occasions. It has been suggested, according to the IV International Workshop on Asymptomatic Primary Hyperparathyroidism, that an isolated measurement of PTH above the upper limit of the normal range should be confirmed on at least two further occasions over a period of 3–6 months [8]. It also has to be noted that in some patients with traditional pHPT, the classical biochemical hallmark of hypercalcemia is not always present and they may also have normal serum and ionized calcium levels at times during their disease course. It is important to distinguish these patients from those with NCpHPT, in which the serum calcium is always normal over the entire course of monitoring. On the other hand, we have to follow-up patients with NCpHPT with serum calcium measurement because they may develop hypercalcemia.
6.8 Conclusion and Therapeutic Implications
Our task in this chapter was to describe the changing clinical presentation of pHPT. Nowadays, asymptomatic pHPT largely prevails upon the classic symptomatic form and a new clinical entity named NCpHPT due to improved diagnostic capability that is emerging. This recognition is useful from a diagnostic standpoint for clinicians, who should not overlook biochemical markers, such as mild hypercalcemia, in the face of inappropriately raised serum PTH levels. Moreover, the detection of persistently increased serum PTH levels and normal calcium levels (both total calcium corrected for albumin levels and ionized calcium) should lead to additional investigation to ascertain the diagnosis of NCpHPT (Fig. 6.1). Of utmost importance, the clinical characterization of the individual pHPT patient with a complete assessment for specific organ damage is mandatory for defining the therapeutic approach [17, 35–37].
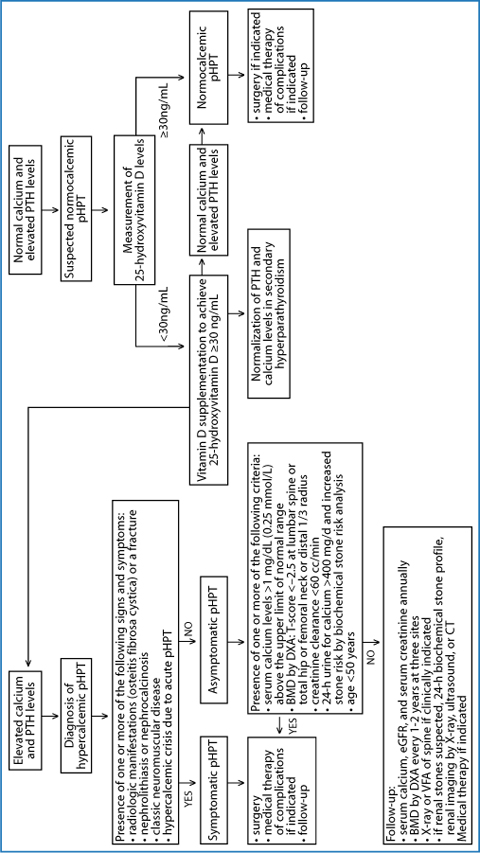

Fig. 6.1
Biochemical and clinical characteristics of different forms of primary hyperparathyroidism with current recommended therapeutic approach and follow-up. BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; eGFR, estimated glomerular filtration rate; pHPT, primary hyperparathyroidism; PTH, parathyroid hormone; VFA, vertebral fracture assessment
It is well acknowledged that PTx is the only curative intervention for patients with pHPT if no contraindication exists. However, surgical operation should be performed in symptomatic pHPT patients and in asymptomatic ones, with at least one of the following features according to the Proceedings of the Fourth International Workshop [17]:
serum calcium levels >1 mg/dL (0.25 mmol/L) above the upper limit of normal range;
T-score BMD by DXA <-2.5 at lumbar spine, total hip, femoral neck or distal 1/3 radius;
a vertebral fracture detected by X-ray, CT, MRI or VFA;
creatinine clearance <60 cc/min;
24-h urine for calcium >400 mg/dL and increased stone risk by biochemical stone risk analysis;
presence of nephrolithiasis or nephrocalcinosis detected by X-ray, ultrasound or CT;
age <50 years.
Asymptomatic patients who do not meet any criteria for PTx should perform DXA at three sites every 1–2 years and should evaluate glomerular filtration rate, serum creatinine and serum total calcium, annually [17]. Moreover, if clinically indicated (height loss, back pain), patients should perform X-ray or VFA of spine; while 24-h biochemical stone profile, renal imaging by X-ray, ultrasound, or CT are indicated in the case of suspected renal stones [17].
Currently no formal and specific guidelines cover NCpHPT. Recent recommendations suggest monitoring calcium and parathormone levels annually and measuring BMD every 1–2 years. If NCpHPT progresses to overt hypercalcemic disease, then guidelines on hypercalcemic pHPT [7] should be referred to. If some complications, such as kidney stones, nephrocalcinosis, fragility fractures or worsening of BMD, occur during the follow-up, then the patient should be referred for surgery [7].
When pHPT is diagnosed during pregnancy, surgery must be performed preferably during the second trimester, in order to avoid the increased risk for fetal complications.
Beside the surgical resolution, it could be necessary to undertake a medical therapy of complications such as osteoporosis, nephrolithiasis and symptoms related to moderate or severe hypercalcemia [35, 36].
Medical therapy of osteoporosis in pHPT is based on the use of an antios-teoporotic agent (oral alendronate or risedronate) together with general measure, such as physical activity as well as adequate calcium intake and cautious vitamin D supplementation. Vitamin D should be given in modest amounts to reach a minimum serum level of 25-OH-D >20 ng/dL (50 nmol/L). Because there is some evidence that levels >30 ng/mL may be associated with further reductions in PTH levels, one could make a reasonable argument to aim for this higher threshold value.
In the follow-up, measurement of BMD by DXA every 18–24 months and evaluation of vertebral spine throughout X-ray or VFA by DXA, if indicated, should guide the choice of continuing or stopping bone active agents.
Medical management of nephrolithiasis may include: adequate hydration (at least 2 liters/day) to maintain a high urinary output, thereby decreasing urinary supersaturation; regular physical activity to minimize bone resorption and normal calcium dietary intake (up to 1000 mg/day). Moreover, low salt and animal protein intake should be indicated for preventing stone formation. pHPT patients with symptomatic nephrolithiasis should be referred for urological evaluation. Patients with recurrent symptomatic episodes should undergo metabolic testing to assess whether stones are caused by systemic disease and if prophylactic treatment should be considered. If feasible, passed stones should be analyzed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree