Fig. 1.1
Types of neuroendocrine neoplasms according to structure, system or organ of insurgence. LCNEC large cell neuroendocrine carcinoma, SCLC small cell lung carcinoma, GEP gastroenteropancreatic tract. World Health Organization classification of tumors – blue book series; *: digestive system (2010) [4]; lung, pleura, thymus, and heart (2015) [7]; endocrine organs (2004) [1]; **: head and neck (2005) [2]; female reproductive organs (2014) [6]; breast (2012) [5]; urinary system and male genital organs (WHO 2016) [8]; skin (2006) [3]
It is well understood that NENs largely retain the specific and peculiar antigen asset of the organ or structure where they originate. Briefly, this means that a paraganglioma arising in the lung or the digestive tract usually does not express epithelial markers of differentiation (e.g., cytokeratin) while retaining vimentin expression, a broad marker of mesenchymal signature. Vice versa, cytokeratin expression is usually retained in NENs arising in the anterior pituitary, the lung, the gut, the pancreas, and the endocrine organs. As expected for cancer, exceptions do exist and may confuse this relatively simple rule though are relatively well described in the mentioned WHO pathology reference blue books.
The shared neural antigen expression refers to the so-called general markers of neuroendocrine differentiation which include the membrane-associated neural adhesion molecule (N-CAM, CD56), the cytosol-associated markers neuron-specific enolase (NSE) and PGP 9.5, the large dense core vesicle (LDCV)-associated chromogranins (including the most popular chromogranin A), and the small synaptic-like vesicle (SSV)-associated synaptophysin [14]. All these antigens are known to have variable degrees of specificity and sensitivity in NENs that should be taken into due account for diagnostic purposes. In addition, the so-called specific markers of neuroendocrine differentiation are also found in NENs, though with a tissue-specific distribution that reflects the distribution of the normal cell counterpart [14]. As exemplification the expression of insulin identifies the beta cell of the islets of the pancreas and is almost invariably expressed only in NEN arising in the pancreas, mostly functioning and thus defined as insulinomas. Again rare exceptions of non-pancreatic insulinomas do exist [15].
NENs have a typical morphology, no matter their site of origin and their name (Fig. 1.2). The structure is “organoid” with either trabecular, acinar, glandular components that may be variably represented and admixed. The tumor cells are usually monomorphic with variably represented either polygonal epithelial-like or fusiform mesenchymal-like types, low or almost undetectable mitotic activity and low proliferation fraction as measured by Ki-67 index. These morphological aspects are consistent with a well-differentiated morphology, usually also defining a low-grade NEN. In some specific anatomical location and, namely, in the adrenal and other paraganglia, NEN cells may display abnormal features that are unequivocally identified as severe atypia, though always with low mitotic and Ki-67 indexes. Such aspects are considered as regressive features and are known possible exception to the usual, typical morphology. The bland features and low proliferation activity of the majority of NENs are the morphological counterpart of their bland clinical behavior which makes NENs slow-pace malignant neoplasms with fairly long/excellent prognosis.
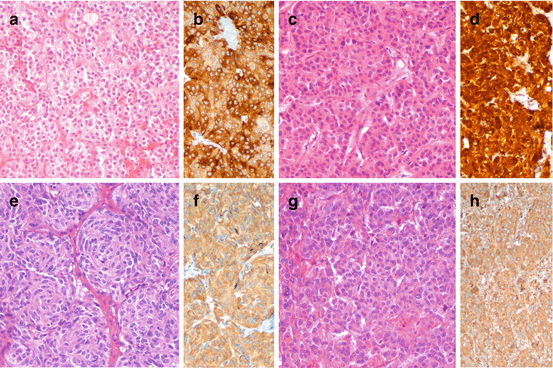

Fig. 1.2
Morphology of low-grade, well-differentiated NEN in pituitary (a, prolactin-producing adenoma, b prolactin immunoreactivity), thyroid (c, calcitonin-producing medullary thyroid carcinoma, d calcitonin immunoreactivity), lung (e, typical carcinoid, f chromogranin A immunoreactivity), and adrenal (g, pheochromocytoma, h chromogranin A immunoreactivity). a, c, e, g hematoxylin and eosin; b, d, f and h immunoperoxidase
A consistent fraction of NENs are still characterized by organoid structure though with more solid aspects, moderate cell atypia with detectable mitotic activity or proliferation fraction by Ki-67 (Fig. 1.3) and possible necrosis though in focal spots. These aspects connote what has been variably defined as atypical NENs. The atypical morphology of NENs may often be accompanied by other standard aggressive feature including invasion of vessels and perineural spaces as well as of surrounding structures, suggesting a potentially different (more aggressive) clinical behavior. This morphology, though largely overlapping the well-differentiated morphology of the typical NEN, probably identifies a condition of well to moderate differentiation and usually defines NENs of intermediate grade. Indeed it is now well understood that for some anatomical site (e.g., the respiratory system and the digestive tract and pancreas), increased proliferation activity either measured by mitotic count or Ki-67 proliferation index with or without overt atypical morphology associates with still relatively slow-pace but surely more aggressive clinical behavior [16, 17].
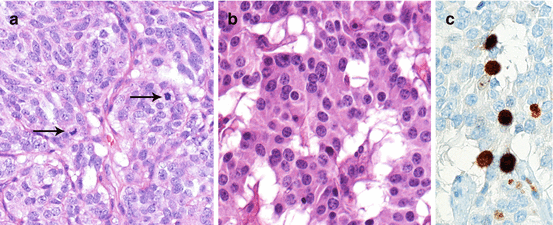

Fig. 1.3
Morphology of low-/intermediate-grade, well-intermediately differentiated NEN in lung (a, atypical carcinoid; note the mitoses [arrows]) and pancreas (b, neuroendocrine tumor; Ki-67 16 % corresponding to a NET G2). a, b hematoxylin and eosin; c immunoperoxidase
Finally a fraction of NENs, usually small at most sites but quite frequent in the lung, display very aggressive morphological features, characterized by sort of organoid but prevalently solid structure with large areas of necrosis, often defined as geographical chart necrosis, and severe cell atypia with brisk mitotic activity, atypical mitoses, and elevated proliferation fraction by Ki-67 (Fig. 1.4). Cell types are either of small type with little cytoplasm and fine nuclear chromatin or large type with abundant cytoplasm, spotted chromatin, and prominent nucleoli. These morphological features are quite different from the typical and, at large extent also, the atypical NEN morphology, are consistent with poor tumor cell differentiation, and are usually defined high-grade NENs. Their clinical behavior is invariably highly malignant and the prognosis is very poor.
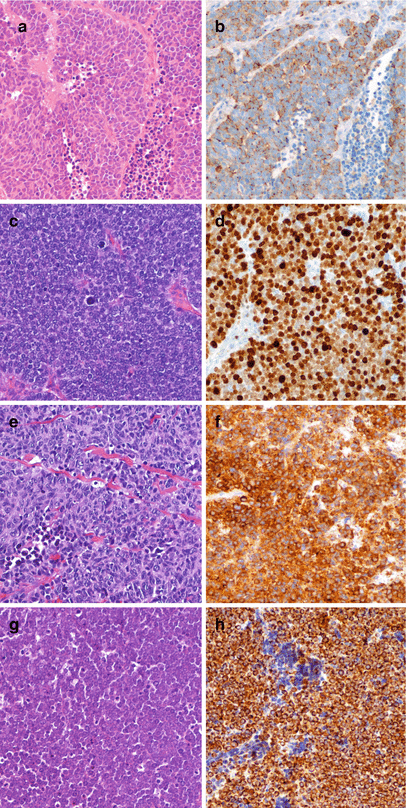

Fig. 1.4
Morphology of high-grade poorly differentiated NEN in lung (a, small cell carcinoma, b chromogranin A immunoreactivity), stomach (c, small cell carcinoma, d Ki-67 nuclear immunoreactivity), bladder (e, small cell carcinoma, SmCC, f synaptophysin immunoreactivity), skin (g, small cell carcinoma, h cytokeratin 20 immunoreactivity). a, c, e, g hematoxylin and eosin; b, d, f, h immunoperoxidase
Overall such morphological features would describe three steps of NEN cancer aggressiveness better defined as grades and as such identified in the lung and the digestive tract and pancreas. This is a common theme for NENs overall, although the presence and incidence of each of such three types is quite variable and different at different anatomical location (Table 1.1). In addition, when solely considering morphology, it appears that two NEN cancer types are probably represented, one including the NENs with typical and atypical morphology that are both rather close each other and a second one with poorly differentiated morphology, rather different versus typical and atypical NENs. Clinical and morphological features connecting morphologically typical and atypical NENs are certainly evident, easily observed, and relatively frequent. By converse clinical and morphological features blending morphologically atypical and poorly differentiated NENs are rare.
Table 1.1
Neuroendocrine neoplasm definitions according to the current WHO classifications
Anatomical site | Classification definitions | Classification reference | ||
|---|---|---|---|---|
Low grade | High grade | |||
Well differentiated | Moderately differentiated a | Poorly differentiated | ||
Pituitary | Typical adenoma (monomorph, rare mitoses, low Ki-67) | Atypical adenoma (invasive growth, high mitoses and Ki-67; no metastases) Pituitary carcinoma (atypical features; metastasis) | nd | WHO classification of Tumors of Endocrine Organs 2004 |
Thyroid | Medullary thyroid carcinoma | nd | WHO classification of Tumors of Endocrine Organs 2004 | |
Parathyroid | Adenoma (encapsulated neoplasm, usually single gland) carcinoma (invasion of vessels, perineural space, capsule, adjacent tissues and/or metastases) | nd | WHO classification of Tumors of Endocrine Organs 2004 | |
Lung and thymus | Typical carcinoid (carcinoid morphology and <2 mitoses/2 mm2; no necrosis) | Atypical carcinoid (carcinoid morphology and 2–10 mitoses/2 mm2 and/or necrosis) | Small cell lung carcinoma (SCLC) Large cell NE carcinoma (LCNEC) (small or large cells; >10 mitoses/2 mm2 and necrosis)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |