Advancement in the understanding of lung tumor biology enables continued refinement of lung cancer classification, reflected in the recently introduced 2015 World Health Organization classification of lung cancer. In small biopsy or cytology specimens, special emphasis is placed on separating adenocarcinomas from the other lung cancers to effectively select tumors for targeted molecular testing. In resection specimens, adenocarcinomas are further classified based on architectural pattern to delineate tissue types of prognostic significance. Neuroendocrine tumors are divided into typical carcinoid, atypical carcinoid, small cell carcinoma, and large cell neuroendocrine carcinoma based on a combination of features, especially tumor cell proliferation rate.
Key points
- •
Lung cancer classification strives to correlate tumor cell morphology with tumor biological characteristics, thus facilitating therapeutic decision-making and effective prognostic outcome prediction in the era of personalized medicine.
- •
In small biopsy specimens or cytology specimens, major types of lung cancers are established by morphologic evaluation, that is, adenocarcinoma and squamous cell carcinoma.
- •
When poorly differentiated carcinomas are encountered, judicious application of immunohistochemical stains facilitates such distinction in most cases.
- •
In resection specimens, lung adenocarcinomas are further divided into low-grade (lepidic adenocarcinoma), intermediate-grade (acinar and papillary adenocarcinomas), and high-grade (solid and micropapillary adenocarcinomas) types of prognostic significance.
- •
Analysis of neuroendocrine tumors is initiated by the recognition of neuroendocrine morphology, verified by neuroendocrine marker expression when necessary.
Introduction
Significant progress has been made in the understanding of lung cancer biology, due in large part to advancement in the understanding of tumor biology and pathogenesis. Acquisition of key somatic mutations acts as a sentinel event in lung carcinogenesis, essential for tumor cell growth and division. Molecular detection of driver mutations in specific histologic types of lung cancer can predict favorable response to targeted therapy. The essence of personalized medicine is to tailor individual lung cancer treatment based on accurate histologic classification and biomarker information. Therefore, characterization of histologic type of lung cancer plays an increasingly pivotal role in the multidisciplinary approach in the diagnosis and management of lung cancer. Recognizing the biological diversity of lung cancer, a comprehensive and accurate tumor classification has been developed, which is important for treatment and prognosis. Pathology of lung cancer has expanded to cover both tissue diagnosis and selection of specific subtypes of lung cancers for further molecular testing. Confirmatory histologic diagnosis directs surgical resection of early-stage disease, whereas pathologic classification and molecular testing enable selection of tumor type–tailored adjvuant therapy and genotype-based treatment regimen to improve the survivals of advanced-stage patients.
Lung cancers are traditionally divided into non–small cell carcinoma (NSCC) and small cell carcinoma (small cell lung carcinoma, SCLC), with the former accounting for 80% of the cases and the latter accounting for the remaining 20%. SCLCs behave aggressively and are treated nonsurgically in most cases, whereas NSCCs are managed by a combination of surgery and adjuvant therapy. Recognition of the diversity of NSCC has led to its subclassification, culminating in the 2004 and 2015 World Health Organization (WHO) classifications. Major types of NSCC include adenocarcinoma, squamous cell carcinoma (SSC), and large cell carcinoma (LCC). Thus, subtype of NSCC is specified, whereas the designation “NSCC” is only preserved in certain small biopsies and cytology specimens. SCLC is grouped with other tumors exhibiting neuroendocrine differentiation. Since the publication of the last volume, significant update in lung cancer classification has occurred for lung adenocarcinomas based on better understanding of tumor biology. This update is manifested by streamlined classification for small biopsies and cytology specimens, with special emphasis on separating adenocarcinomas from the rest of the lung cancers in order to effectively screen cases responsive to current mutation-driven therapeutic paradigms. More detailed histologic subtyping is used in resection specimens to delineate tissue types of prognostic significance. This article discusses current pathologic classification of lung cancer, with an emphasis on updating readers to the new WHO lung adenocarcinoma classification ( Box 1 ). This article thus serves as a springboard for effective surgical and medical treatment modalities discussed in other articles in this series.
Adenocarcinoma
Lepidic adenocarcinoma
Acinar adenocarcinoma
Papillary adenocarcinoma
Micropapillary adenocarcinoma
Solid adenocarcinoma
Invasive mucinous adenocarcinoma
Colloid adenocarcinoma
Fetal adenocarcinoma
Enteric adenocarcinoma
Minimally invasive adenocarcinoma
Squamous cell carcinoma
Neuroendocrine tumors
Carcinoid tumors
Typical carcinoid
Atypical carcinoid
Small cell carcinoma
Large cell neuroendocrine carcinoma
Large cell carcinoma
Adenosquamous carcinoma
Pleomorphic carcinoma
Spindle cell carcinoma
Giant cell carcinoma
Carcinosarcoma
Pulmonary blastoma
Other and unclassified carcinomas
Lymphoepithelioma-like carcinoma
NUT carcinoma
Salivary gland–type carcinomas
Mucoepidermoid carcinoma
Adenoid cystic carcinoma
Epithelial-myoepithelial carcinoma
Mesenchymal tumors, lymphohistiocytic tumors, tumors of ectopic origin, and metastatic tumors
Adenocarcinoma
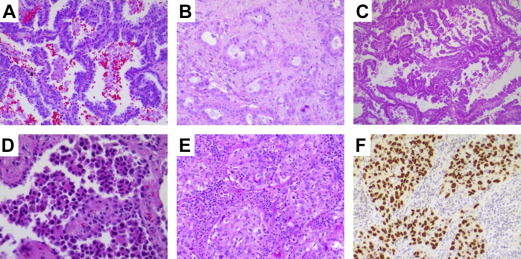
Adenocarcinoma is the most common type of lung cancer, accounting for more than 40% of lung cancers, 60% of the NSCC, and more than 70% of surgically resected cases. The incidence of adenocarcinoma has risen steadily over the past few decades. Lung adenocarcinoma commonly forms a peripherally located mass with central fibrosis and pleural puckering. It can also have a variety of other gross appearances, including centrally located mass, diffuse lobar consolidation, bilateral multinodular distribution, and pleural thickening. By definition, lung adenocarcinoma is a malignant epithelial neoplasm with glandular differentiation or mucin production. When such morphologic features are recognized, the tumor can be designated as adenocarcinoma, even in small biopsy specimens. Lung adenocarcinoma cells usually express pneumocytic markers. Thyroid transcription factor (TTF-1) and NapsinA are expressed in more than 85% of the lung adenocarcinoma cases and thus can serve as markers of adenocarcinoma or adenocarcinoma differentiation in poorly differentiated tumor and in limited biopsy sampling material ( Fig. 1 ). Tumor classification based on ancillary tests such as immunohistochemistry (IHC) is designated as “NSCC, favor adenocarcinoma” in a small biopsy specimen. Resection specimens allow a more detailed subclassification. There has been significant refinement in adenocarcinoma classification in recent years based on close pathologic and clinical correlation. The major histologic types have been validated to bear prognostic significance delineated by the tumor grade. Multiple gene alterations can occur in adenocarcinomas, with approved molecular targeted therapy available to improve patient survival (see later disccusion).
Preinvasive or Minimally Invasive Adenocarcinoma
Adenocarcinoma in situ (AIS) represents relatively small sized tumors (≤3 cm) with neoplastic cells growing along preexisting alveolar structures (lepidic growth pattern) without evidence of stromal, vascular, or pleural invasion. Lepidic is a descriptive term for rind or membranous growth pattern and is now specifically used to describe tumor cells proliferating along the surface of intact alveolar walls. Lepidic growth usually correlates with ground glass opacity on radiograms. Most AISs are the nonmucinous type, with mild to moderate pleomorphic cuboidal to columnar tumor cells linearly lining alveolar walls. There is no secondary papillary or micropapillary growth pattern. A minority of such tumors are of mucinous or mixed type. If the tumor contains a small focus (<5 mm) of invasive growth, the tumor is classified microinvasive adenocarcinoma (MIA). Invasion usually induces formation of a desmoplastic stroma. Invasion can also manifest as nonlepidic growth, such as acinar, papillary, micropapillary, or solid patterns. MIA is defined not only by limited size invasive growth but also by a lack of more advanced invasive pattern, such as tumor necrosis, lymphovascular invasion, and pleural invasion. Both types of tumor are low grade and have a nearly 100% 5-year survival rate.
Invasive Adenocarcinoma
Most invasive adenocarcinomas are composed of mixed morphological subtypes; these are classified according to the predominant architectural structures rather than lumped together as mixed subtype. Each tumor is classified according to one predominant growth pattern, including lepidic, acinar, papillary, micropapillary, and solid patterns (see Fig. 1 ). Each additional subpattern is recorded semiquantitatively as estimated percentage in 5% increments. This architectural-driven classification has prognostic significance, with most favorable prognosis for lepidic-predominant adenocarcinomas, intermediate survival rate for acinar and papillary predominant adenocarcinomas, and poor prognosis for solid and micropapillary predominant adenocarcinomas.
Lepidic Adenocarcinoma
Lepidic growth is commonly seen in lung adenocarcinoma. The lepidic growth pattern denotes tumor cells spreading along preexisting alveolar structures, although there may be sclerotic thickening of alveolar septa. When it is the predominant growth pattern with additional findings that set it apart from previously described AIS and MIA, it is designated as lepidic adenocarcinoma. These additional findings include any one or more of the following: more than 5 mm of invasion (presence of desmoplastic or myofibroblastic stroma); spread through air spaces; lymphatic or vascular invasion; pleural invasion; tumor necrosis. Although such tumors were previously classified as bronchioloalveolar carcinoma, this term is no longer used because it encompasses a heterogeneous group of adenocarcinomas. This category convenes a significantly better prognosis than other subtypes.
Acinar Adenocarcinoma
Acinar adenocarcinoma is a common type of adenocarcinoma with tumor cells arranged in classic glandular structure on a fibroelastic stroma. It is important to separate demosplastic stroma in this pattern from preexisting alveolar structures with thickened fibroelastic alveolar septa sometimes seen in a lepidic pattern. It is of note that the tumor cells displaying more complex growth patterns, such as a cribriform pattern, likely represent a poor prognostic subtype convening a significant risk of recurrence.
Papillary Adenocarcinoma
The tumor cells form papillary architecture with tumor cells lining the surface of branching fibrovascular cores. The presence of fibrovascular cores separates this tumor type from micropapillary adenocarcinoma.
Micropapillary Adenocarcinoma
The tumor cells form individual cellular tufts without fibrovascular core. The tumor cells appear as detached small and solid individual cell groups. Psammoma bodies may be seen. This subtype of adenocarcinoma has distinctly poor prognosis in the early stage compared with other subtypes of adenocarcinoma.
Solid Adenocarcinoma
The tumor cells form patternless sheets and lack any other recognizable patterns, including poorly differentiated/undifferentiated carcinomas expressing pneumocytic markers (such as TTF-1 and NapsinA), which were formerly grouped in the LCC category. It is of note that certain markers commonly associated with squamous cell differentiation, such as p63, and less commonly, p40, can show focal expression in solid adenocarcinoma.
Rare Variants of Invasive Adenocarcinoma
Rare variants of invasive adenocarcinoma include invasive mucinous adenocarcinoma, colloid and fetal adenocarcinoma, and enteric adenocarcinoma. Invasive mucinous adenocarcinoma is frequently multicentric and the tumor cells lack expression of TTF-1 commonly seen for lung adenocarcinoma. Enteric adenocarcinoma should be distinguished from metastatic colorectal adenocarcinoma.
Adenocarcinoma
Adenocarcinoma is the most common type of lung cancer, accounting for more than 40% of lung cancers, 60% of the NSCC, and more than 70% of surgically resected cases. The incidence of adenocarcinoma has risen steadily over the past few decades. Lung adenocarcinoma commonly forms a peripherally located mass with central fibrosis and pleural puckering. It can also have a variety of other gross appearances, including centrally located mass, diffuse lobar consolidation, bilateral multinodular distribution, and pleural thickening. By definition, lung adenocarcinoma is a malignant epithelial neoplasm with glandular differentiation or mucin production. When such morphologic features are recognized, the tumor can be designated as adenocarcinoma, even in small biopsy specimens. Lung adenocarcinoma cells usually express pneumocytic markers. Thyroid transcription factor (TTF-1) and NapsinA are expressed in more than 85% of the lung adenocarcinoma cases and thus can serve as markers of adenocarcinoma or adenocarcinoma differentiation in poorly differentiated tumor and in limited biopsy sampling material ( Fig. 1 ). Tumor classification based on ancillary tests such as immunohistochemistry (IHC) is designated as “NSCC, favor adenocarcinoma” in a small biopsy specimen. Resection specimens allow a more detailed subclassification. There has been significant refinement in adenocarcinoma classification in recent years based on close pathologic and clinical correlation. The major histologic types have been validated to bear prognostic significance delineated by the tumor grade. Multiple gene alterations can occur in adenocarcinomas, with approved molecular targeted therapy available to improve patient survival (see later disccusion).
Preinvasive or Minimally Invasive Adenocarcinoma
Adenocarcinoma in situ (AIS) represents relatively small sized tumors (≤3 cm) with neoplastic cells growing along preexisting alveolar structures (lepidic growth pattern) without evidence of stromal, vascular, or pleural invasion. Lepidic is a descriptive term for rind or membranous growth pattern and is now specifically used to describe tumor cells proliferating along the surface of intact alveolar walls. Lepidic growth usually correlates with ground glass opacity on radiograms. Most AISs are the nonmucinous type, with mild to moderate pleomorphic cuboidal to columnar tumor cells linearly lining alveolar walls. There is no secondary papillary or micropapillary growth pattern. A minority of such tumors are of mucinous or mixed type. If the tumor contains a small focus (<5 mm) of invasive growth, the tumor is classified microinvasive adenocarcinoma (MIA). Invasion usually induces formation of a desmoplastic stroma. Invasion can also manifest as nonlepidic growth, such as acinar, papillary, micropapillary, or solid patterns. MIA is defined not only by limited size invasive growth but also by a lack of more advanced invasive pattern, such as tumor necrosis, lymphovascular invasion, and pleural invasion. Both types of tumor are low grade and have a nearly 100% 5-year survival rate.
Invasive Adenocarcinoma
Most invasive adenocarcinomas are composed of mixed morphological subtypes; these are classified according to the predominant architectural structures rather than lumped together as mixed subtype. Each tumor is classified according to one predominant growth pattern, including lepidic, acinar, papillary, micropapillary, and solid patterns (see Fig. 1 ). Each additional subpattern is recorded semiquantitatively as estimated percentage in 5% increments. This architectural-driven classification has prognostic significance, with most favorable prognosis for lepidic-predominant adenocarcinomas, intermediate survival rate for acinar and papillary predominant adenocarcinomas, and poor prognosis for solid and micropapillary predominant adenocarcinomas.
Lepidic Adenocarcinoma
Lepidic growth is commonly seen in lung adenocarcinoma. The lepidic growth pattern denotes tumor cells spreading along preexisting alveolar structures, although there may be sclerotic thickening of alveolar septa. When it is the predominant growth pattern with additional findings that set it apart from previously described AIS and MIA, it is designated as lepidic adenocarcinoma. These additional findings include any one or more of the following: more than 5 mm of invasion (presence of desmoplastic or myofibroblastic stroma); spread through air spaces; lymphatic or vascular invasion; pleural invasion; tumor necrosis. Although such tumors were previously classified as bronchioloalveolar carcinoma, this term is no longer used because it encompasses a heterogeneous group of adenocarcinomas. This category convenes a significantly better prognosis than other subtypes.
Acinar Adenocarcinoma
Acinar adenocarcinoma is a common type of adenocarcinoma with tumor cells arranged in classic glandular structure on a fibroelastic stroma. It is important to separate demosplastic stroma in this pattern from preexisting alveolar structures with thickened fibroelastic alveolar septa sometimes seen in a lepidic pattern. It is of note that the tumor cells displaying more complex growth patterns, such as a cribriform pattern, likely represent a poor prognostic subtype convening a significant risk of recurrence.
Papillary Adenocarcinoma
The tumor cells form papillary architecture with tumor cells lining the surface of branching fibrovascular cores. The presence of fibrovascular cores separates this tumor type from micropapillary adenocarcinoma.
Micropapillary Adenocarcinoma
The tumor cells form individual cellular tufts without fibrovascular core. The tumor cells appear as detached small and solid individual cell groups. Psammoma bodies may be seen. This subtype of adenocarcinoma has distinctly poor prognosis in the early stage compared with other subtypes of adenocarcinoma.
Solid Adenocarcinoma
The tumor cells form patternless sheets and lack any other recognizable patterns, including poorly differentiated/undifferentiated carcinomas expressing pneumocytic markers (such as TTF-1 and NapsinA), which were formerly grouped in the LCC category. It is of note that certain markers commonly associated with squamous cell differentiation, such as p63, and less commonly, p40, can show focal expression in solid adenocarcinoma.
Rare Variants of Invasive Adenocarcinoma
Rare variants of invasive adenocarcinoma include invasive mucinous adenocarcinoma, colloid and fetal adenocarcinoma, and enteric adenocarcinoma. Invasive mucinous adenocarcinoma is frequently multicentric and the tumor cells lack expression of TTF-1 commonly seen for lung adenocarcinoma. Enteric adenocarcinoma should be distinguished from metastatic colorectal adenocarcinoma.
Squamous cell carcinoma
SCCs make up about 20% of lung cancers. Their incidence has declined in recent decades, likely because of changes in smoking behavior. SCC usually occurs in the central portion of the lung, along major airways, and can form cavities when it achieves a large size. On microscopic examination, SCC characteristically shows keratinization and intercellular bridges and exhibits a solid nested growth pattern ( Fig. 2 ). The tumor cells usually have hyperchromatic nuclei, visible to inconspicuous nucleoli, and moderate to abundant cytoplasm with delineated intercellular bridges. There can be individual tumor cell keratinization or groups of keratinizing squamous cells forming keratin pearls centrally placed within solid tumor nests. The tumor cells lack glandular structure or mucin production. SCCs are further divided into keratinizing, nonkeratinizing, and basaloid subtypes. Contrary to adenocarcinoma subtypes, such subclassification shows no apparent prognostic utility except for basaloid SCCs, which reportedly display distinct molecular profile conferring intrinsic resistance to cytotoxic chemotherapy. Recognizing morphologic features of squamous cell differentiation, including keratinization, keratin pearl formation, and intercellular bridges, establishes SCC diagnosis, even in small biopsy specimens. When the tumor is poorly differentiated and does not allow confident morphologic classification, selective squamous cell markers, such as p40, CK5/6, CK5, and p63, are used to demonstrate squamous differentiation (see Fig. 2 ). TTF-1 stain is usually negative, although focal weak positive stain for this marker has been reported. Poorly differentiated tumor defined by expression of squamous cell markers in a biopsy material is diagnosed as “NSCC, favor SCC.” Although tumors in this category are usually excluded from current molecular testing, identification of driver mutations in SCC, such as discoidin domain receptor 2 (DDR2), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PI3KCA), fibroblastic growth factor receptor 1 (FGFR1), and v-akt murine thymoma viral oncogene homolog 1 (AKT1), may enable future personalized therapy. Establishment of squamous cell differentiation has important implications in chemotherapeutic agent choices so as to avoid certain complications. For instance, treatment with the vascular endothelial growth factor inhibitor bevacizumab in patients with SCC can potentially precipitate life-threatening pulmonary hemorrhage, and therefore, should be avoided. Survival rate for SCC is significantly better than adenocarcinoma.
Large cell carcinoma
LCCs represent a minority of NSCC cases that are devoid of lineage-specific differentiation, and lack morphologic and immunohistochemical evidence of adenocarcinoma, SCC, or neuroendocrine carcinoma (null immunophenotype). LCC is usually peripherally located, bulky, and necrotic in appearance. The tumor cells are large and polygonal in shape with pleomorphic and vesicular nuclei. The tumor cells form patternless solid sheet or nests. Large cell neuroendocrine carcinoma (LCNEC) is classified in the lung neuroendocrine tumor (NET) category (see later discussion). LCCs represent less than 3% of the lung cancers. Tumors with morphologic characteristic of LCC are designated as “NSCC, not otherwise specified (NOS)” in a biopsy or cytology material, not LCC, because the latter can only be ascertained in a resection specimen with the thoroughly analyzed tumor devoid of lineage-specific differentiation. It is important to apply ancillary tests such as IHC to avoid inclusion of poorly differentiated NSCC, such as solid adenocarcinoma and nonkeratinizing SCC, in this category. In fact, most LCCs defined by morphologic criteria can be reclassified as adenocarcinoma and SCC using a panel of lineage-specific markers. Effective application of such ancillary tests is the likely explanation for the decline of lung cancers categorized as LCC in recent years. Recent evidence suggests that tumors currently classified as LCC with null immunophenotype share molecular characteristics similar to solid adenocarcinoma. Tumors in this category are not necessarily excluded from molecular testing. Metastatic tumors should be excluded in appropriate clinical context. Most LCC cases have adverse outcome, especially for those with null immunophenotype.
Other non–small cell carcinoma types
Adenosquamous carcinoma is a rare type of NSCC, accounting for less than 5% of all lung cancers. It represents a hybrid carcinoma containing both adenocarcinoma and SCC components, with each comprising at least 10% of the tumor. From a clinical prospective, adenosquamous carcinoma is usually included in the discussion of adenocarcinomas because therapeutically important mutations occur in adenosquamous carcinoma in similar frequency as in conventional adenocarcinoma ; this is especially significant in light of its worse prognosis than that of adenocarcinoma and SCC.
Pleomorphic, spindle cell, and giant cell carcinomas are rare types of NSCC, accounting for less than 3% of lung cancers. These tumors demonstrate spindle and/or giant cell differentiation (sarcoma-like differentiation). Such tumors can be ascertained only in resection specimens. In biopsy specimens, tumors with such features are reported as “NSCC with spindle and/or giant cell carcinoma.” Expression of cytokeratin is important in this setting to exclude a primary pulmonary sarcoma. Because some tumors can show weak or even absent expression of common epithelial markers, multiple anticytokeratin antibodies may be necessary. Epithelial markers commonly used include pancytokeratin, cytokeratin AE1/AE3, CK7, and EMA. Such tumors have a worse prognosis than conventional NSCC.
Carcinosarcoma is composed of NSCC and sarcomatous elements, such as rhabdomyosarcoma, chondrosarcoma, and osteosarcoma. Pulmonary blastoma must be distinguished from pleuropulmonary blastoma occurring in the pediatric population. Carcinomas can rarely arise from bronchial glands, analogous to salivary gland tissue. Major histologic types include mucoepidermoid carcinoma, adenoid cystic carcinoma, and epithelial-myoepithelial carcinoma.
Neuroendocrine tumors
NETs as a group are relatively common lung tumors, accounting for about 20% to 25% of lung cancers. Their common morphologic, immunohistochemical, and ultrastructural features set them apart from other lung tumors. These features include organoid growth pattern, finely granular or “salt-and-pepper” chromatin pattern, and the expression of several hallmark neuroendocrine markers. Common neuroendocrine markers include chromogranin A, synaptophysin, and CD56. Within this group of tumors, there is a heterogeneous degree of differentiation, with well-differentiated tumors retaining most or all of the above characteristics and poorly differentiated tumors losing some or most of the discernible neuroendocrine differentiation features. This histologic differentiation is epitomized by tumor proliferation rate, which in turn correlates with tumor aggressiveness and prognosis. The 2015 WHO classification separates this group of tumors into 4 major categories, including typical carcinoid, atypical carcinoid, small cell carcinoma (or SCLC), and LCNEC ( Table 1 ). Because tumor cell proliferation rate has been shown to provide accurate overall prognostic information, this has been used along with the presence or absence of necrosis to divide NETs into 3 grades of prognostic significance. The low-grade NET corresponds to typical carcinoid; intermediate-grade NET corresponds to atypical carcinoid, and high-grade NET corresponds to SCLC and LCNEC. The proliferation rate is expressed as the number of mitoses per microscopic unit area of tumor (usually defined as mitoses per 2 mm 2 or 10 high-magnification microscopic fields). In recent years, utilization of Ki67 labeling index as an adjunct for grading has gained popularity. This Ki67 labeling index is especially useful in evaluating small biopsy specimens, whereby it is often impossible to count adequate microscopic fields to give an accurate reflection of proliferation rate.
| Typical Carcinoid | Atypical Carcinoid | SCLC | LCNEC | |
|---|---|---|---|---|
| Neuroendocrine morphology | Monotonous cells arranged in organoid nesting, palisading, rosettes, trabeculae | Monotonous cells arranged in organoid nesting, palisading, rosettes, trabeculae | Small cell size, finely granular chromatin, inconspicuous nucleoli, scanty cytoplasm | Large cell size, frequent presence of nucleoli, abundant cytoplasm |
| Mitoses per 2 mm 2 | <2 | 2–10 | >10, usually >60 | >10, usually >30 |
| Ki67 proliferation index | ≤4–5% | ≤20–25% | >50% | Usually >40% |
| Necrosis | No | Focal or punctuate | Often extensively present | Often extensively present |
| Neuroendocrine marker expression | Yes | Yes | Yes, rarely negative | Yes |
| Grade | Low grade | Intermediate grade | High grade | High grade |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree