Chronic Obstructive Pulmonary Disease: Introduction
The smoking epidemic of the twentieth century has led to an increase in the incidence of chronic obstructive pulmonary disease (COPD), a largely preventable disease. The statistics concerning COPD have caused considerable alarm around the world. Globally, COPD is the fourth leading cause of mortality and the twelfth leading cause of disability. To address this growing problem, the World Health Organization partnered with the National Heart, Lung, and Blood Institute (NHLBI) to form a Global Initiative for Chronic Obstructive Lung Disease (GOLD). In 2001, they offered a global strategy to increase awareness of the disease and offer guidelines for disease prevention and treatment, referred to as the GOLD Guidelines. These guidelines and those created by leading medical societies are incorporated in this chapter.
Most patients are diagnosed with COPD in the sixth decade. Although it is an important chronic disease and a leading cause of disability in the elderly, it remains underrecognized. This chapter will focus on the early recognition and management of COPD, an important component of outpatient geriatric management.
Definitions
GOLD defines COPD as partially reversible or nonreversible airflow limitation, which is progressive, and cannot be reversed by current therapies. In contrast asthma is defined as a syndrome characterized by reversible airflow limitation. Although GOLD definitions did not include traditionally used terminologies, such as chronic bronchitis and emphysema, these definitions are important to understand the disease spectrum. Chronic bronchitis is defined clinically as cough with sputum production for 3 months of a year for 2 consecutive years. Emphysema is a pathological diagnosis defined as the destruction of alveolar walls with accompanying enlargement of air spaces distal to the terminal bronchiole.
Key differences between COPD and asthma are that, in COPD there is (i) a lack of complete reversibility of airflow obstruction; (ii) neutrophil predominance in the airways, especially in the lumen; (iii) significant smoking history (usually >10 pack years) or exposure to burning biomass fuel, such as wood and manure; (iv) chronic colonization of bacterial organisms in the airways, especially in patients with severe disease; and (v) emphysematous changes in the lung parenchyma often associated clinically with reduced diffusing capacity on a gas diffusion test.
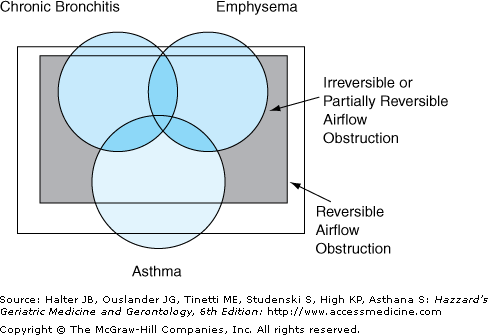
It is important to recognize that some of these distinctions become blurred in the elderly. Firstly, many elderly asthmatics, even those who have never smoked, have evidence of poorly reversible airflow obstruction, similar to COPD. This is caused by permanent remodeling of the airways. Secondly, bronchial hyperresponsiveness, an exaggerated bronchoconstrictive response to a given stimulus, is seen in a majority of middle-aged smokers with COPD and is a strong predictor of progressive decline in lung function. Thirdly, adults with asthma, especially those with severe disease, often demonstrate neutrophilia in their airways. Lastly and perhaps most importantly, many adult patients with asthma are current or former smokers. It is likely that such patients have more than one pathological process and several pathways of inflammation. These patients are likely to have both COPD and asthma. This has raised a complexity of semantic issues that has not been resolved. One attempt has been to combine and describe such patients using the term asthmatic bronchitis (Figure 83-1).
An important limitation of current definitions of COPD is that they fail to identify a precise level of airflow limitation at which clinically relevant outcomes occur for each age group. This is an important issue in the elderly, in whom reduced elastic recoil of lung with aging maybe associated with increased incidence of airways obstruction. Using a commonly used criteria, such as forced expiratory volume in 1st second/forced vital capacity (FEV1/FVC) ratio <70% may thus lead to over diagnosis. Using age-specific cutoffs of FEV1/FVC ratio to diagnose COPD is therefore important in the elderly.
Epidemiology
The different definitions of COPD, based on spirometry, clinical, or radiographic criteria, have important implications when estimating the global burden of disease. Using patient- or physician-reported diagnosis alone may underestimate the incidence of COPD. More than 50% of individuals with airway obstruction have never been diagnosed with COPD by a health care provider. Underdiagnosis is more common in the elderly because they fail to report symptoms or symptoms are attributed to other conditions.
Several recent studies used multiple approaches, including spirometry and clinical criteria, to estimate the prevalence of COPD. In the United States, the National Health and Nutrition Examination Survey (NHANES) III estimated prevalence of COPD from 1988 to 1994. In this survey, 14.3% of the adult population or 24.2 million individuals had airflow limitation by spirometry. Of these, 1.5% had moderate to severe disease, evidenced by FEV1 <50%. However, only 2.9% or 4.8 million adults who met spirometry criteria report symptoms of chronic bronchitis or emphysema.
In the NHANES III survey, the prevalence of airflow obstruction increased with age and was highest among those 65 to 85 years of age. The prevalence of low lung function is higher with increasing age except in individuals 85 years or older, which maybe related to differential mortality or inability to do pulmonary function testing. This survey also underscored the difficulties in differentiating asthma and COPD. More than a fifth of the respondents reported that they had current asthma and current bronchitis or had current asthma and current emphysema.
Similar estimates have been obtained globally. For instance, a prevalence study in Japan estimated the prevalence of COPD in those 40 years or older to be 10.9% by spirometry criteria. In the Platino Study, which evaluated residents 40 years of age or older in five major Latin American cities (Sao Paulo, Santiago, Mexico City Montevideo, and Caracas), the prevalence estimates ranged from a low of 7.8% in Mexico City to a high of 19.7% in Montevideo. Similar estimates have been reported in European countries.
COPD is a leading cause of death worldwide. Globally, COPD is the fourth leading cause of mortality and the twelfth leading cause of disability. In 2004, chronic lower respiratory diseases, including chronic bronchitis and emphysema, were the fourth leading cause of death in United States and it is estimated that approximately 6% of deaths occur as a result of COPD. Patients with COPD often die as a result of influenza or pneumonia, the sixth leading cause of death in United States. These findings combined with the underdiagnosis of COPD suggests that current COPD mortality estimates maybe underestimated.
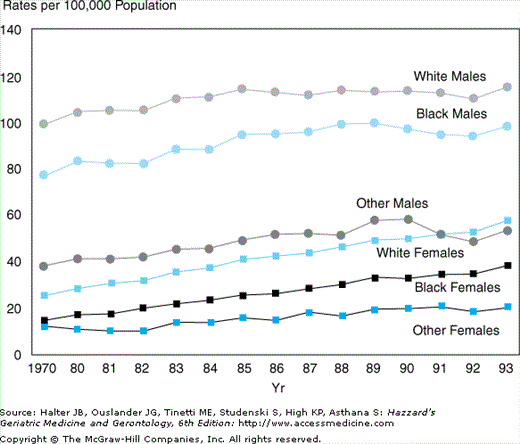
An alarming trend has been increasing mortality as a result of COPD over the past two decades. In United States, mortality caused by COPD has increased 44% from 1979 to 1993, and the highest increase in mortality has been experienced by women (Figure 83-2). This is in sharp contrast to the declining mortality caused by cardiovascular disease and cancer. Worldwide it is estimated that by the year 2020, COPD will be the third leading cause of death and the fifth leading cause of disability.
Although accurate statistics regarding morbidity as a result of COPD are difficult to obtain, it represents an important cause of disability. The average number of days of restricted activities reported by patients with COPD is very high. In 1996, COPD was listed as the eighth leading cause of disability-adjusted life-years (DALYs) in men, and the seventh leading cause of DALYs among women. COPD is a leading cause of hospitalizations in United States. In 1998, nearly 2% of all hospitalizations were attributed to COPD and 7% had COPD as a contributing cause of hospitalization. More striking are COPD statistics regarding the elderly population. Nearly 20% of all hospitalizations in patients older than age 65 years had COPD as a primary or contributing cause. Owing to its impact on reduced physical activity, it is emerging as an important cause of reduced functional status and disability in older adults. The cost implications of treating COPD are huge and those with severe disease account for the highest cost.
Pathophysiology
Table 83-1 lists important risk factors for COPD. Exposure to inhalants, including tobacco, indoor and outdoor air pollution, are important environmental risk factors. Cigarette smoking is the most important risk factor for COPD. At least 15% of smokers develop COPD, though recent estimates suggest higher incidence among smokers. Both current and previous smokers are at increased risk of COPD. Pipe and cigar smokers and passive exposure to cigarette smoke also increases risk, though these individuals are at lower risk compared to cigarette smokers. In NHANES III survey, obstructive lung disease was present among 12.5% of current smokers, 9.4% of former smokers, and 3.1% of pipe or cigar smokers. Indoor pollution caused by cooking using biomass fuels is an important risk factor, especially for females in developing countries. The role of urban pollution in susceptibility to COPD is unclear.
RISK FACTOR | COMMENT |
|---|---|
Cigarette smoking | Most important risk factor and most cases of COPD are caused by smoking |
Pipe and cigars | High risk, but lower than observed in cigarette smokers |
Occupational exposure | Risk in coal miners, gold miners, grain handlers, cement and cotton workers |
Environmental pollution | Indoor use of biomass fuels for cooking and heating in underdeveloped countries and particulate matter from urban pollution |
Genetic factor | Alpha-1-antitrypsin deficiency known to cause early onset COPD. Other genetic risk factors may also increase risk |
Socioeconomic | More common with low socioeconomic status |
Childhood illnesses | Low birth weight, respiratory infections, and symptomatic childhood asthma may increase risk |
The vast majority of individuals exposed to cigarette smoke do not develop symptomatic COPD. Thus host factors may explain differences in susceptibility. Genetics, especially gene–environment or gene–inhalant interaction, may play an important role. For instance, several studies in the 1970s observed higher rates of airflow obstruction in first-degree relatives of patients with COPD compared to control subjects. COPD also aggregates in families.
Severe alpha-1 antitrypsin deficiency is an important genetic risk factor and accounts for 1% of COPD patients. Alpha-1 antitrypsin, a serum protein, is synthesized in the liver. It inhibits proteolytic enzymes, such as trypsin, chymotrypsin, and neutrophil elastase. If neutrophil elastases are not inactivated by alpha-1 antitrypsin, these enzymes destroy lung connective tissue, particularly elastin, and cause emphysema. Understanding the role of alpha-1 antitrypsin in COPD has therefore provided a foundation for the protease–antiprotease hypothesis for the pathogenesis of COPD. The phenotype, also called Pi type, is determined by the independent expression of two independent alleles. More than 90% of severely deficient patients are homozygous for the Z allele. Such patients are designated Pi ZZ and have serum alpha-1 antitrypsin levels that are about 20% of the normal level. The phenotypic effects of having a single Z allele, heterozygote with Pi MZ genotype, are less clear. Individuals with alpha-1 antitrypsin deficiency develop COPD during the fourth or fifth decade.
Recent studies have also examined the role of other genes and their interaction with environmental factors to explain differences in susceptibility and outcomes of COPD. Candidate genes include tumor necrosis factor, microsomal epoxide hydrolase, glutathione S-transferases, heme oxygenase-1, and alpha-1-antichymotrypsin. Although preliminary studies suggest that these genetic variants may play an important role in COPD pathogenesis, results have not been consistent across studies.
COPD is characterized by chronic inflammation in peripheral airways and alveoli. Macrophages, neutrophils, and CD8+ T lymphocytes are the predominant cells involved in inflammation. In contrast to asthma, eosinophils are not present in lung biopsy specimens. Important mediators include leukotrience B4, IL-8, and TNF-α. Cigarette smoking and other irritants activate resident macrophages to release chemotactic factors, which attract inflammatory cells from the circulation and release additional inflammatory mediators. The subsequent interaction between the molecular and cellular mediators is complex and poorly understood.
Two theories have been proposed to explain pathogenesis of COPD: protease–antiprotease imbalance theory and increased oxidative stress theory. These theories suggest that lung inflammation is amplified in the presence of excess proteases or oxidant stress. In the protease–antiprotease imbalance theory, proteases, such as elastase, proteinase 3, and matrix metalloproteinases, are induced by cigarette smoking and lead to alveolar wall destruction and mucus hypersecretion. These proteolytic enzymes are counteracted by antiproteases, particularly alpha-1 antitrypsin, and inhibitors of matrix metalloproteinases. The imbalance between proteases and antiproteases may lead to destruction of connective tissue and cause emphysema.
The oxidative stress theory was proposed because of increase in markers of oxidative stress in exhaled breath and urine in COPD patients. Interestingly, the role of oxidative stress has also been implicated in aging. Oxidative stress is caused by the imbalance between reactive oxygen species and the body’s ability to detoxify reactive oxygen species or the damage caused as a result. Excess oxidative stress has several consequences, including activation of inflammatory genes and inactivation of antiproteases, thereby leading to increased local inflammation.
The pathologic changes in COPD are found in the large and small airways and in the lung parenchyma. Structural changes in the airways include mucus hyperplasia, bronchiolar edema and smooth muscle hypertrophy, and peribronchiolar fibrosis. These changes result in narrowing of the small airways. Early pathophysiologic abnormalities in airways that are 2 mm and less in diameter have been referred to as “small-airway disease.” Mucus hypersecretion occurs owing to increase in the size and number of the submucosal glands and an increase in the number of goblet cells on the surface epithelium.
Emphysema is a destructive process that occurs in the gas-exchanging airspaces, the respiratory bronchioles, alveolar ducts, and alveoli. It results in obliteration of airspace walls and coalescence of small distinct air spaces into larger ones. An important consequence of emphysema is loss of elastic recoil of the lungs and thereby increases airway obstruction. Emphysema also causes abnormal gas exchange.
In advanced stages, there are also changes in the pulmonary circulation, heart, and respiratory muscles. Alveolar hypoxia causes medial hypertrophy of vascular smooth muscle with extension of the muscularis layer into distal vessels that do not ordinarily contain smooth muscle. Intimal hyperplasia also occurs in advanced stages. These latter changes are associated with the development of pulmonary hypertension and its consequences, right ventricular hypertrophy. Loss of vascular bed also occurs with emphysema as a result of destruction of alveolar walls. In some patients with advanced COPD, there is atrophy of diaphragmatic muscle.
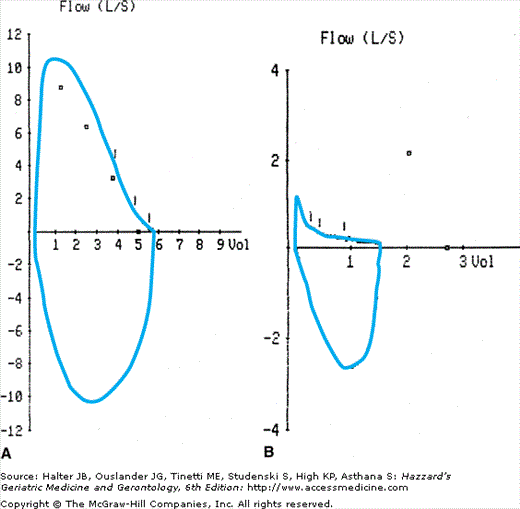
Pathologic processes affecting airways and lung parenchyma are accompanied by changes in respiratory physiology in COPD. The physiologic hallmark of COPD is the limitation of expiratory flow caused by airway narrowing and emphysematous changes in the lung. This is accompanied by a reduction in the FEV1/FVC ratio. Early in the course of COPD, the expiratory flow–volume curve has a scooped out appearance in the expiratory limb as a result of reduced flow at low lung volumes (Figure 83-3). With disease progression, expiratory flow decreases at all lung volumes. Another important effect of decreased expiratory flow is nonuniform ventilation of the lungs, observed even in the earlier stages of COPD. Nonuniform ventilation can lead to ventilation–perfusion mismatches, which reduce arterial oxygen pressures.
Hypercapnia is usually observed in the end-stages of COPD, usually when FEV1 is 1 L or lower. Pulmonary hyperinflation places the thoracic cage and diaphragm at a mechanical disadvantage and increases the work of breathing. Airflow obstruction also contributes to increased work of breathing. Finally, the destruction of lung parenchyma worsens ventilation perfusion mismatch and worsens both hypercapnia and hypoxemia.
There has been increased interest in understanding systemic consequences of COPD for several reasons. First, reduced exercise capacity observed in patient’s with COPD patients is not limited by respiratory system abnormalities alone. Several studies suggest that cardiovascular and skeletal muscle changes also contribute to reduced exercise capacity. Second, most COPD patients die because of nonpulmonary causes, such as cardiovascular disease and cancer. Third, COPD patients have higher prevalence of reduced body mass index (BMI), osteoporosis, and increased systemic concentrations of inflammatory markers. These observations are underscored by a recently proposed multidimensional risk prediction score by Celli et al., in which survival prediction was improved by incorporating BMI, dyspnea scale, and exercise capacity in the traditional risk prediction model using FEV1 alone. Although there is an improved understanding of the systemic consequences of COPD, the biologic mechanisms underlying these effects remain poorly understood.
COPD patients have reduced skeletal muscle strength. Upper extremity grip strength is usually well preserved in COPD, but the strength of the lower extremity quadriceps is reduced compared to age-matched controls. Respiratory muscle strength is also reduced, but it is more likely to occur because of hyperinflation of lung rather than intrinsic defect in the muscles. The decline in skeletal muscle strength in COPD patients is proportional to the reduction in FEV1. During hospitalization for exacerbations, skeletal muscle strength is reduced further, but the long-term effect of an exacerbation on skeletal muscle strength is not known. Thigh muscle size and lower extremity lean body mass is also lower in COPD patients. Multiple factors may contribute to reduced skeletal muscle strength, including elevation of systemic inflammatory markers, particularly tumor necrosis factor or cachectin and IL-6, disuse atrophy, hypogonadism, poor nutrition, and hypoxemia.
COPD is also associated with changes in body composition. These patients have lower BMI and lean body mass. However, visceral fat appears to be similar to age-matched control population. Advanced COPD patients have higher risk of osteoporosis as a result of smoking, concomitant vitamin D deficiency, low BMI, hypogonadism, sedentary lifestyle, and use of glucocorticoids. The risk of osteoporosis is often underrecognized until the first fracture occurs.
There is a high prevalence of cognitive impairment in COPD. The most common disturbances are in verbal fluency, memory tasks, attention, and deductive thinking. Cognitive impairment in COPD has been related to age, educational background, depression, disability, exercise tolerance, and duration of respiratory failure. Although it is related to hypoxia, it does occur in nonhypoxemic COPD patients. Relationships between fatigue, depression, and cognitive function are poorly documented.
Clinical Manifestations
Most clinical manifestations occur during the fifth and sixth decade of life. However, the onset of COPD symptoms maybe delayed to the seventh decade and beyond in smokers who stopped smoking in midlife. Dyspnea is the most common symptom of COPD and an important reason for reduced physical activity. Initially dyspnea is present only on exertion, but as lung function declines, patients experience dyspnea at rest too. Similar to dyspnea, chronic productive or nonproductive cough is intermittent initially and becomes persistent in severe disease. Chronic intermittent nonproductive cough can be present for several years prior to onset of dyspnea and maybe a subtle manifestation of mild COPD. Wheezing or chest tightness usually occurs with exertion.
An initial assessment of a COPD patient should include past medical history of asthma, detailed history of smoking and occupational exposures, family history of early onset COPD, and frequency of exacerbations or hospitalizations. Some symptoms may also be related to coexisting chronic health conditions, such as cardiovascular disease, which are common in these patients. Furthermore, COPD patients may develop secondary hypertension (cor pulmonale) that manifests as worsening dyspnea and lower extremity swelling. When patients develop rapid worsening of symptoms, alternative diagnoses should be considered, such as exacerbation of underlying disease, pneumothorax, or pulmonary thromboembolism.
Physical examination findings in the respiratory system are present in advanced disease and include barrel-shaped chest on inspection, prolonged expiration, distant heart sounds, and rhonchii on auscultation. Patients often use accessory muscles or pursed lips during breathing. Signs of cor pulmonale include pedal edema, a tender congested liver, loud pulmonary component of the second heart sound, and jugular venous distention. Careful attention should be paid to systemic manifestations, including cognitive deficits, reduced muscle strength, and recent weight loss.
Functional status assessment is important in COPD. Subjective and objective evaluations, based on activities of daily living and a 400-meter or a 6-minute walk test, is important to monitor disease progression and are independent predictors of mortality. Oxygen desaturation during an informal walk test in the office setting can identify patients who will benefit with supplemental oxygen.
Diagnosis
Underdiagnosis or misdiagnosis of COPD is common in the elderly because symptoms of dyspnea and reduced functional status are often attributed to aging or coexisting disease. Therefore, spirometry, which includes an FEV1 and FVC measurement with a flow volume loop, should be performed when COPD is suspected. A full pulmonary function test (PFT), which includes FEV1, FVC, lung volumes, and diffusion capacity, can be performed in selected patients.
The peak expiratory flow (PEF) meter is often used to monitor disease in asthma. The PEF meter measures airflow obstruction in the larger airways. Since pathological changes occur predominantly in the smaller airways in COPD, the PEF meter underrepresents the severity of airflow obstruction in COPD, and therefore, should not be used for diagnosis or disease monitoring.
Challenges to perform spirometry in geriatric patients include difficulties in comprehension of instructions, dexterity problems, and reduced functional capacity. These problems often lead to poor quality results and are an important deterrent to performing spirometry in the elderly. Simple office-based spirometry tests using handheld spirometer are becoming more reliable and can be done in the outpatient office setting. These devices are easy to use and less demanding, and therefore, maybe particularly valuable in the diagnostic evaluation of older adults with suspected COPD.
A spirometer is a device that measures lung volumes through the forced expiration of air. Flow can also be assessed, by measuring the total volume of air that is expired in the first second of expiration. There are well-defined normal ranges that allow for the effects of age, race, sex, and height. Three measurements from the spirometer are commonly used in the diagnosis of COPD. FEV1 measures the air exhaled in the first second of expiration. FVC is the maximum volume of air that can be forcibly expired. The ratio of FEV1/FVC is expressed as a percentage and is the proportion of total volume of air that can be expired in the first second of expiration.
Spirometry results will only be of value if the tracings are performed satisfactorily and consistently. Two criteria should be met before results of spirometry are interpreted. First, the tracings should be reproducible, where at least two of the three FEV1 measurements should be within 100 mL or 5% of each other. Second, the results should meet the acceptability criteria, where the measurements must continue until no more air can be exhaled. Exhalation is prolonged in COPD because of expiratory flow limitation and can take up to 15 seconds. Acceptability criteria can be assessed by examining the volume–time curve and at least 6 seconds of exhalation should be present or the exhalation should plateau for the last 2 seconds. Frequent coughing during spirometry may affect results.
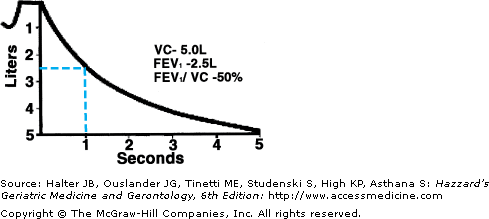
COPD is diagnosed based on the FEV1/FVC ratio less than 70% (Figures 83-3 and 83-4). However, in older adults, especially those over 70 years, this cutoff will overestimate disease. Therefore, age-adjusted ratios are preferred, though are cumbersome to use. However, for most older adults, a ratio below 60% is abnormal and suggestive of obstructive lung disease. Once obstruction is diagnosed, severity is graded based on the FEV1 measurement (Table 83-2). The presence of reduced FEV1, but a normal FEV1/FVC ratio, is suggestive of restrictive lung disease, such as idiopathic pulmonary fibrosis. These findings should prompt referral for a full PFT and pulmonary consultation.
GLOBAL INITIATIVE FOR CHRONIC OBSTRUCTIVE DISEASE (GOLD) | AMERICAN THORACIC SOCIETY (ATS)/EUROPEAN RESPIRATORY SOCIETY (ERS) | |
|---|---|---|
At risk | Risk factors and chronic symptoms but normal Spirometry | – |
Mild | FEV1/FVC<70% FEV1≥80% predicted value | FEV1≥70% predicted value |
Moderate | FEV1/FVC<70% 80%>FEV1≥50% predicted value | 70%>FEV1≥60% predicted value 60%>FEV1≥59% predicted value (moderately severe) |
Severe | FEV1/FVC<70% 50%>FEV1≥30% predicted value | 49%>FEV1≥35% predicted value |
Very severe | FEV1/FVC<70% FEV1≤30% predicted value | FEV1<35% predicted value |
Improvement in FEV1 in response to bronchodilators, termed bronchodilator reversibility, is based on increase in FEV1 by 12% and 200 mL. Though bronchodilator reversibility suggests the diagnosis of asthma, many elderly subjects with asthma do not demonstrate response to bronchodilators. In individuals with an obstructive pattern on spirometry without evidence of airway reversibility, other clinical findings should be taken into account to differentiate asthma versus COPD. However, this distinction is often difficult in the elderly. It is important to emphasize that bronchodilator reversibility of findings on spirometry correlates poorly with clinical response to short or long-acting bronchodilators.
Bronchial or airway hyperresponsiveness is the exaggerated bronchoconstrictive response to nonspecific agonists, such as methacholine, hypertonic saline, adenosine, exercise, and hyperventilation. Although it is a hallmark of asthma, it can be present in several individuals with COPD and is associated with worse prognosis. Routine assessment of bronchial hyperresponsivess in elderly patients has limited value in clinical practice currently.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree