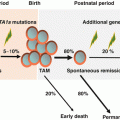
Idiopathic AA
Secondary AA
Hepatitis associated
Secondary (drug/chemical/radiation/infection)
Inherited AA
Fanconi anemia
Dyskeratosis congenita
Shwachman-Diamond syndrome
Congenital amegakaryocytic thrombocytopenia
Others
Myelodysplastic syndrome
Refractory cytopenia of childhood (provisional)
Refractory cytopenia with multilineage dysplasia
Refractory anemia with excess blast
Other diseases entity
Hemophagocytic lymphohistiocytosis
Acute leukemia
Bone marrow invasion of solid tumor
Autoimmune
Nutritious deficiency
Myelofibrosis
Hypersplenism
Table 6.2
Severity grading of childhood AA and evaluation criteria to IST
Severity grading of AA [19] | Nuetrophil | Platelet | Reticulocyte | ||
|---|---|---|---|---|---|
Moderate | At least 2 levels | <1000/μL | <5 × 104/μL | <6 × 104/μL | |
Severe | At least 2 levels | <500/μL | <2 × 104/μL | <2 × 104/μL | |
Very severe | <200/μL | <2 × 104/μL | <2 × 104/μL | ||
Evaluation criteria to IST [111] | Neutrophil | Platelet | Hb | Transfusion | |
Severe/very severe | Complete response | ≧1500/μL | ≧10 × 104/μL | ≧11.0 g/dL | No |
Partial response | ≧500/μL | ≧3 × 104/μL | ≧8.0 g/dL | No | |
No response | <500/μL | ||||
Non-severe AA | Complete response | ≧1500/μL | ≧10 × 104/μL | ≧11.0 g/dL | No |
Partial response | ≧1000/μL | ≧3 × 104/μL | ≧8.0 g/dL | No | |
No response | <1000/μL |
6.3.2 Distinguishing Inherited AA from Other Types of AA
Because the optimal management differs considerably between inherited AA and other types of AA, correct and timely diagnosis is crucial. However, no useful test for such differentiation is available, except for diagnosing Fanconi anemia. The diagnosis of inherited AA is difficult because of clinical and genetic heterogeneity. Autosomal recessive inheritance, de novo mutations, and genetic anticipation often mask familial accumulation. In addition, the BM morphology of inherited AA varies and may change over time; therefore, BM examination should be repeated to evaluate the cellularity, dysplasia, monoclonality, and leukemic changes. Because such patients are at high risk of developing MDS/AML and solid tumors, genetic diagnosis is mandatory for subsequent medical management.
6.3.3 Distinguishing AA from Myelodysplastic Syndrome
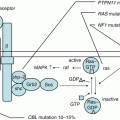
The distinction between AA and MDS is the presence of dysplasia or increased numbers of blast cells. The World Health Organization classification (2008) for MDS added the provisional entity of refractory cytopenia of childhood (RCC), defined as hypocellular marrow with mild dysplasia in one or two lineages with blast cells <5% [20]. However, a few experts agree that AA can be discriminated from refractory cytopenia with multilineage dysplasia (RCMD) and refractory anemia with excess blasts (RAEB) but not from RCC [21]. According to the reevaluation of BM morphology included in the Japanese prospective studies (AA-92 and AA-97), the response to IST, 5-year overall survival (OS), and the incidence of clonal abnormalities were comparable among AA, RCC, and RCMD groups [8]. A recent German study also reported the efficacy of IST for RCC (the response rate at 6 months, 74% in horse-ATG group [hATG] and 53% in rabbit-ATG group [rATG]) [22]. In addition, intensive genetic analyses including 88 IBFMS-associated genes and 96 myeloid malignancy-related genes in children with IAA/RCC/RCMD (n = 168) revealed that the somatic mutation frequency did not differ among AA, RCC, and RCMD patients [23]. BCOR and PIGA mutations were repeatedly identified. Importantly, MDS-related gene mutations such as U2AF1 plus SETBP1 and TP53 mutations were found in only two patients with RCMD.
These findings suggest that AA and RCC have a common clinical and molecular-pathological nature, while the molecular pathogenesis in RCMD is heterogeneous and may progress to advanced disease.
6.4 Standard Treatment
6.4.1 Treatment Algorithm for vSAA or SAA (Fig. 6.1)
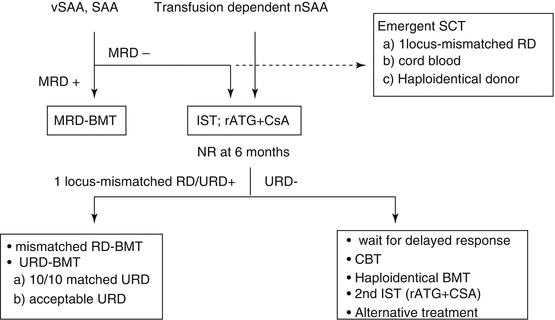
Fig. 6.1
Treatment algorism for children with SAA and vSAA. vSAA very severe aplastic anemia, SAA severe aplastic anemia, nSAA non-severe aplastic anemia, MRD matched related donor, BMT bone marrow transplantation, IST immunosuppressive therapy, rATG rabit anti-thymocyteglobulin, CSA cyclosporine, CBT cord blood transplantation, NR non renponse, URD unrelated donor, SCT stem cell transplantation
- 1.
If a matched related donor (MRD) is available, immediate bone marrow transplant (BMT) is indicated.
- 2.
If a MRD is not available, IST with rATG and CSA is indicated.
- 3.
If no response is obtained at 6 months after IST, BMT from an alternative or unrelated donor (URD) is indicated.
Recent reviews provide a similar treatment algorithm for childhood AA [24–27]. Figure 6.1 shows the current minimum consensus for vSAA and SAA. However, this needs to be modified based on the availability and accessibility of diagnostic technology and resources (drug and donor source). Indeed, hATG (ATGAM®) is not available in some countries including Japan. Volunteer donors and cord blood are also limited, and the coordination time for URD varies among countries.
According to the updated results comparing MRD-BMT and IST in Japanese children, failure-free survival (FFS) is significantly lower in the IST group compared with the BMT group (56% vs. 87%, respectively) [28]. Similar results were reported in a recent European study, which found that FFS was 33% for IST but 87% for up-front MRD-SCT [29].
The incidence of relapse after the first IST using hATG + CSA was 11–35% in children [30–32]. Although patients who relapse after a primary response to IST are likely to respond to a second IST, multiple courses of IST are associated with an increased incidence of Epstein-Barr virus-associated lymphoproliferative disorder (EB-LPD) after the subsequent SCT [33].
A Japanese prospective study to compare the efficacy of repeated IST with BMT from alternative donors in children with SAA who were refractory to the first IST showed that the 5-year FFS from the second-line therapy was 84% in the alternative donor BMT group but 10% in the second IST group. Therefore, alternative donor BMT is recommended for children refractory to the first IST [34].
BM is the preferred stem cell source in both MRD-SCT and URD-SCT. The use of peripheral blood stem cells (PBSC) is one of the significant risk factors for survival, acute graft-versus-host disease (aGVHD) and chronic graft-versus-host disease (cGVHD) [35–38]. In exceptional cases with a high risk of graft failure, the use of PBSC may be justified. In developing countries, several reports showed that PBSC was associated with less graft failure and comparable survival rate compared with BM graft [39, 40].
6.4.2 Transplantation from Matched Related Donor (Tables 6.3 and 6.4)
In Japan, various conditioning regimens have been used in MRD-BMT [41]. Currently, cyclophosphamide (CY, 200 mg/kg) plus rATG (Thymoglobulin®) is the standard conditioning regimen, with successful engraftment rates with low toxicity [42]. The standard for GVHD prophylaxis comprises CSA (3 mg/kg/day, intravenously) and short-course methotrexate (sMTX) (15 mg/m2 on day 1, then 10 mg/m2 on days 3, 6, and 11) [43, 44]. The target trough level of CSA is 100–200 ng/mL in the whole blood. Slow weaning from CSA should be started at least 12 months after MRD-BMT. In Western countries, the higher trough level of CSA (150–250 ng/mL) is recommended for 9–12 months after transplantation [25].
Table 6.3
Outcomes of stem cell transplantation in childhood aplastic anemia (recent selected studies)
Group | Age | Number | Donor source | OS (5 years) | Graft failure (primary + late) | Acute GVHD (II-IV) | Chronic GVHD | Conditioning regimen | GVHD prophylaxis | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
Japan | Children | 434 | Related | 93% | Various | Various | [90] | |||
298 | Unrelated | 88% | ||||||||
26 | Cord blood | 73% | ||||||||
Japan | Children | 30 | Related | 100% | 3% | 0% | 3% | CY (200 mg/kg) + ATG, CY (200 mg/kg) + TLI, Flu (100 mg/m2) + CY(100 mg/kg) + TLI | CSA + MTX | [41] |
31 | Unrelated | 94% | 9% | 37% | 27% | CY (120-200 mg/kg) + TBI (5-10Gy) + ATG | CSA/FK + MTX | |||
Korea | Children | 23 | Related + unrelated | 90% | 4% | 26% | 9% | Flu (120 mg/m2) + CY (100 mg/kg) + ATG | CSA + MTX | [112] |
USA | Children | 15 | Related | 93% | 7% | 0% | 14% | CY (200 mg/kg) + ATG/BU (only for PNH) | CSA/FK +/− MTX | [113] |
23 | Unrelated | 89% | 4% | 17% | 17% | CY (200 mg/kg) + TBI (2-4Gy) + ATG/alemtuzumab +/− CA | ||||
UK | Children | 44 | Unrelated | 95% | 0% | 2% (III-IV) | 12% | Flu (150 mg/m2) + CY (120-200 mg/kg) + alemtuzumab | CSA, CSA + MMF/MTX, FK | [60] |
UK/EBMT | Children | 29 | Upfront, unrelated | 96% | 3% | 10% | 19% | Flu (150 mg/m2) + CY (120 mg/kg) + alemtuzumab +/− TBI (2-3Gy) | CSA +/− MMF | [82] |
EBMT | Children | 396 | Upfront, related | 91% | 2% | 8% | 6% | Various | Various | [29] |
91 | Post-IST, related, unrelated, mismatched related | 83% | 2% | 25% | 20% | |||||
China | Children | 20 | Haploidentical (2–3 loci mismatch) | 80% | 5% | 58% | 17% | Flu (150 mg/m2) + CY (120-200 mg/kg) + ATG +/− TBI (2-5Gy) +/− BU (2–6.4 mg/kg) | CSA + MTX +/− MMF, FK + MTX | [83] |
Table 6.4
Outcomes of IST with CSA + ATG as a front-line therapy in childhood AA (selected studies)
Dosage and usage | Results | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Study group | Formulation | Patients | N | Severity | rATG | CSA (oral) | Response at 6 months | OS | Relapse | Reference |
Japan | rATG | Children | 40 | nSAA, SAA, vSAA | 3.5 mg/kg × 5 | 6 mg/kg/day | 48% | 94% | 9% | [114] |
Japan/Korea/China | rATG | Children | 158 | SAA, vSAA | 2.3–5 mg/kg × 5 | 6 mg/kg/day | 55% | 87% | 6% | [68] |
China | rATG | Children | 46 | SAA, vSAA | 2.5 mg/kg × 5 | 5–8 mg/kg/day | 65% | 85% | 5% | [115] |
Taiwan | rATG | Children | 20 | SAA, vSAA | 2 mg/kg × 8 | 5 mg/kg/day | 80% | 90% | 5% | [116] |
EWOG | rATG | Children | 32 | SAA, vSAA | 3.75 mg/kg × 5 | 5 mg/kg/day | 34% | 92% | nd | [117] |
UK | rATG | Children | 43 | SAA, vSAA | 3.75 mg/kg × 5 | 5 mg/kg/day | 33% | 94% | 31% | [60] |
Poland | rATG | Children | 63 | SAA, vSAA | 3.75 mg/kg × 5 | 5 mg/kg/day | 49% | 67% | 12% | [118] |
Brazil | rATG | Children | 26 | SAA, vSAA | 5 mg/kg × 5 | 10 mg/kg/day | 35% | 74% | 27% | [119] |
Japan | hATG (Lymphoglobulin®) | Children | 82 | nSAA | 55% | 98% | 35% | [30] | ||
149 | SAA | 61% | 82% | 13% | ||||||
210 | vSAA | 61% | 82% | 12% | ||||||
Germany | hATG (Lymphoglobulin®) | Children | 49 | SAA | 44%a | 81% | nd | [120] | ||
97 | vSAA | 69%a | 92% | nd | ||||||
Italy | hATG (Lymphoglobulin®) | Children | 40 | nSAA, SAA, vSAA | 60%b | 83% | 16% | [32] | ||
USA | hATG (ATGAM®) | Children | 77 | SAA, vSAA | 74% | 80% | 33% | [31] | ||
A prolonged interval between diagnosis and transplantation and no ATG administered during the conditioning regimen are significant risk factors for survival [36, 45]. In addition, prior to IST as well as numerous transfusions and alloimmunization are strongly associated with graft rejection and increased risk of mixed chimerism [46]. Therefore, when MRD is available, BMT should be performed as soon as possible after diagnosis. For such high-risk patients, low-dose radiation could be justified based on the report of a low incidence of late malignancy in a Japanese study [47]. A new regimen comprising fludarabine (Flu, 120 mg/m2), CY (120 mg/kg), and rATG has been widely used in adults with excellent outcomes [48]. Higher incidence of aGVHD and cGVHD was demonstrated in CY+ radiation group compared with CY+ ATG group [49]. The superiority of radiation or alemtuzumab-containing regimens has not been demonstrated in up-front MRD-SCT [36, 38, 49].
6.4.3 Transplantation from Unrelated Donor (Tables 6.3 and 6.4)
Early studies of URD-BMT resulted in poor survival rates of 28–54% in patients with AA [50, 51]. Prior infection due to prolonged aplasia, graft rejection, and severe GVHD as well as increased toxicities caused by the myeloablative regimen, including high-dose total body irradiation (TBI), the presence of anti-HLA antibody, and organ damage by iron overload, may result in poor outcomes. From the late 1990s onward, an early indication for URD-BMT, the introduction of new HLA-typing technology and fludarabine-based non-myeloablative regimens has greatly improved the survival rate, which is currently not inferior to that of MRD-BMT in developed countries [52, 53]. However, URD grafts still have a significant impact on the incidence and severity of aGVHD and cGVHD.
Variable doses of TBI and CY have been used in several clinical trials. Low-dose TBI (2 Gy) improved survival when added to ATG and CY (200 mg/kg total dose) in children with SAA [54]. A CY dose de-escalation study showed that 100 mg/kg or 50 mg/kg of CY combined with Flu (120 mg/m2), ATG, and low-dose TBI (2 Gy) resulted in favorable survival (92–85%) [55, 56].
Based on these findings, Japan Childhood Aplastic Anemia Study Group has recommended a conditioning regimen with CY (3000 mg/m2), Flu (100 mg/m2), rATG (5 mg/kg), and low-dose TBI (3 Gy). Because AA is a benign disorder, all forms of GVHD are detrimental. In particular, cGVHD causes organ dysfunction and secondary malignancy, thus worsening quality of life in the long term. Although a direct comparison between CSA and tacrolimus has not been made, tacrolimus with sMTX has been widely used instead of CSA in Japan, and a retrospective paired-match analysis indicated the superiority of tacrolimus to CSA in URD-BMT [57]. Tacrolimus (0.02 mg/kg/day) is usually used intravenously. The level of tacrolimus is maintained in the range of 10–15 ng/mL in the whole blood. Tacrolimus should be tapered from 12 months after URD-BMT.
Donor selection is a critical issue for URD-BMT. Although a 10/10 or 8/8 allele-matched donor is desirable, such a high level of matching is not always obtained. Based on recent Japanese analysis, any single mismatch or multiple mismatching limited to HLA-C, DQ, and DRB1 may affect the incidence of aGVHD and cGVHD but not survival [58]. These findings expand the URD pool substantially, thus enabling rapid coordination for URD.
In Europe, Flu (120 mg/m2) + CY (120 mg/kg) + rATG is currently recommended in URD-BMT as well as MRD-BMT for AA [59]. Low-dose TBI can be added in patients >14 years old or with a high risk of rejection. In addition, a UK study explored new regimens in which TBI + ATG was replaced with alemtuzumab to reduce the long-term complications. The Flu (150 mg/m2) + CY (120 mg/kg) + alemtuzumab (FCC) regimen conferred an excellent 5-year FFS of 95% in recipients transplanted from 10/10 allele-matched URD [60]. There were no cases of graft failure, but mixed chimerism was a major concern. Another study showed an engraftment failure rate of 9%, cGVHD rate of 11%, and 5-year OS of 90% in SCT from full-matched URD and other related or mismatched URD [38]. The risk of cGVHD was significantly lower in the alemtuzumab group than in the rATG group. However, a less toxic regimen with Flu (120 mg/m2) + CY (1200 mg/m2) + alemtuzumab resulted in higher graft failure (9.5% for MRD-BMT and 14.5% for URD-BMT) and mixed chimerism in T cell [61].
6.4.4 Immunosuppressive Therapy
ATG is a polyclonal anti-T-cell antibody-rich serum fraction produced by immunizing mammals against human thymocytes. ATG includes several brands with different immunogens, such as hATG (Lymphoglobulin® and ATGAM®) and rATG (ATG-F® and Thymoglobulin®). Pig-ATG is also used in China. For SAA, combination therapy with CSA and ATG is the gold standard IST rather than CSA or ATG alone [62, 63]. The response to IST is classified as complete (CR), partial (PR), or no response (NR) (Table 6.2). Table 6.4 shows the long-term outcomes of clinical studies with standard IST.
In 2009, Lymphoglobulin® was withdrawn from the market worldwide. rATG (Thymoglobulin®) was reported to be effective as a salvage therapy for refractory or relapsed AA [64, 65]. However, the first prospective randomized study demonstrated that both response rate and OS were inferior in the rATG group (Thymoglobulin®) as compared with the hATG group (ATGAM®) (Table 6.5) [66].
Table 6.5
Predictive factors to IST in childhood AA
Significance | Factor | Reference |
|---|---|---|
Favorable | WBC count: <2.0 × 109/L | [121] |
Platelet count <25 × 109/L | [122] | |
Absolute neutrophil count (ANC) <0.2 × 109/L | [120] | |
Absolute reticulocyte count ≥25 × 109/L and absolute lymphocyte count ≥1.0 × 109/L | [123] | |
HLA-DR15 (+) and HLA-DR4 (−) | [124] | |
Presence of PNH clone | ||
Unfavorable | Long interval from diagnosis to IST | |
Short telomere length | ||
Duration of ANC = 0 longer than 2 weeks | [127] |
The following studies using rATG (Thymoglobulin®) have shown controversial results in terms of the response rate and OS compared with hATG (Lymphoglobulin®). A European study analyzing outcomes in 105 age- and disease severity-matched pairs showed that the best response rate was comparable between rATG + CSA (60%) and hATG + CSA (67%) [67]. However, the OS at 2 years in the rATG group (68%) was inferior to that in the hATG group (86%) (P = 0.009). Transplant-free survival also differed significantly (52% for rATG and 76% for hATG). In the larger dataset that included 455 pediatric SAA cohorts from Asian countries, the response rate at 6 months was comparable between the hATG and rATG groups (60% vs. 55%, respectively) [68]. However, the OS in the hATG group was significantly better than that in the rATG group (2-year OS, 96% vs. 87%; 10-year OS, 92% vs. 84%, respectively). Currently, most experts conclude that hATG (ATGAM®) is preferred, but the use of rATG (Thymoglobulin®) is justified. An international prospective study to find the better rATG dose (2.5 mg/kg × 5 days vs. 3.5 mg/kg × 5 days) plus CSA (6 mg/kg) is ongoing in Japan, Korea, and China.
Several clinical and laboratory factors are useful to predict the response to IST. Pretreatment peripheral blood counts and other significant factors are shown in Table 6.6. However, these findings were based on IST using hATG + CSA. Few studies using rATG + CSA reported several predictive factors for the response; SAA rather than vSAA, the higher absolute reticulocyte count and the presence of PNH clone were shown to be favorable [69, 70]. Therefore, the predictive factors must be updated in the prospective studies using rATG + CSA.
Table 6.6
Recommended preparative regimen and GVHD prophylaxis in Japan
Donor | AA | RCC | GVHD prophylaxis |
|---|---|---|---|
HLA-matched sibling | CY (200 mg/kg) | Flu (125 mg/m2) | CSA + sMTX |
rATG (5 mg/kg) | L-PAM (140 mg/m2) | ||
rATG (5 mg/kg)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|