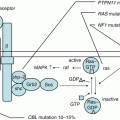
Fig. 13.1
Pathophysiology of infectious mononucleosis (a) and hemophagocytic lymphohistiocytosis (b) due to primary EB virus infection. EBV-IM EB virus-associated infectious mononucleosis, EBV-HLH EB virus-associated hemophagocytic lymphohistiocytosis, INFγ interferon, IL-6 interleukin-6, IL-18 interleukin-18, TNFα tumor necrosis factor-α
13.3 Diagnostic Findings in EBV-HLH
The HLH-2004 criteria have been widely used for the diagnosis of HLH [14]. However, their sensitivity and specificity should be validated before application of these diagnostic criteria is suitable for EBV-HLH. Because these criteria focus on FHL, some of them, including impaired NK cell activity, may not be suitable for EBV-HLH [2]. Increased titers of EBV-specific antibodies are not reliable for the diagnosis of EBV-HLH [2]. Although EBV-HLH sets in following the first EBV infection, there are few cases in which onset is brought on by the reactivation of EBV accompanied by chronic active EBV infection. In addition, there are few cases of FHL onset triggered by EBV infection. The real-time polymerase chain reaction (PCR) to quantify EBV DNA in the peripheral blood is a useful method for diagnosis of EBV-HLH. Patients with EBV-HLH show a significantly higher viral load compared to IM in both plasma and peripheral blood mononuclear cells on real-time PCR [15]. In addition, CD5 downregulation in CD8-positive T cells may be rapidly discriminated between EBV-HLH and EBV-IM [16].
The monoclonality of EBV-infected T lymphocytes is confirmed in EBV-HLH. The monoclonality of EBV-infected T cells can be evaluated by assessing the uniformity of the EBV genome and/or the monoclonality of T-cell receptor (TCR) gene rearrangement using Southern blotting and conventional PCR analyses [17, 18]. Recently, the multiplex PCR for detecting clonal immunoglobulin and TCR gene rearrangement was established to detect clonally proliferating EBV-containing T-cells in EBV-HLH [19]. The combination of these genetic analyses is useful in the diagnosis of EBV-HLH.
13.4 Prognostic Factors for EBV-HLH
Kogawa et al. [18] reported that factors for poor prognosis were 7 days or more until diagnosis, a neutrophil count of 1700/μL or higher, a prothrombin time/international normalized ratio of 1.68 or higher, an activated partial thromboplastin time (aPTT) of 69 s or more, a total bilirubin (T.Bil) content of 1.8 mg/dL or higher, and lactate dehydrogenase (LDH) level of 4310 IU/L or higher. Prognosis for cases with a T.Bil of 1.8 mg/dL and higher and a ferritin level of 20,300 ng/mL or higher was especially poor. Another study reported that hyperbilirubinemia, hyperferritinemia, and cerebrospinal fluid pleocytosis at diagnosis and thrombocytopenia and hyperferritinemia 2 weeks after the initiation of treatment adversely affect the outcome of HLH [20]. Imashuku et al. [21] reported that despite the high prevalence of abnormal test findings that are found in EBV-HLH and are similar to those in tumors such as EBV clonality, T-cell clonality, and chromosomal abnormality, only chromosomal abnormality can be a factor for poor prognosis. Another study in children and adults with EBV-HLH reported that an adult age, EBV reactivation, and multidrug chemotherapy were associated with a poor clinical outcome in patients with EBV-HLH [2]. Although several clinical factors provide useful information for treatment of EBV-HLH, a larger prospect study is needed.
13.5 Treatment for EBV-HLH and Prognosis
In FHL, etoposide is considered necessary in controlling the disease [22]. Imashuku et al. [23] showed that the overall survival (OS) of the group that used etoposide within 4 weeks of diagnosis reached 90% and that the early use of etoposide is a prognostic factor (use within 4 weeks resulted in an OS of 90.2 ± 6.9% and after 4 weeks resulted in an OS of 56.5 ± 12.6%). Based on the above results, an 8-week treatment with etoposide (HLH-94/HLH2004) has been used in EBV-HLH as the standard treatment. However, other reports indicate that about half of the patients with EBV-HLH can be treated without etoposide. Shiraishi et al. [24] reported that 14 out of 22 cases of EBV-HLH (64%) recovered without etoposide [24]. Those cases that recovered without etoposide had a short period of fever prior to the treatment or had significantly low LDH and sIL-2R values. In a retrospective national survey, 37 out of 93 cases (40%) recovered from EBV-HLH without using etoposide. Three-year OS and event-free survival rates for all EBV-HLH cases were 91.2% and 79.3%, respectively, and there was no difference between etoposide and non-etoposide groups [18]. Therefore, with an etoposide-containing regimen, care should be taken to avoid overtreatment of some EBV-HLH patients. In addition, onset of secondary leukemia has been reported in a case of EBV-HLH that was treated with etoposide [25]; thus, minimizing the administration dose of etoposide is a major challenge.
Allogeneic SCT has decreased the mortality in EBV-HLH patients, but there is insufficient evidence to suggest that SCT is better than immunochemotherapy in children with EBV-HLH [26]. When the toxicity is considered, HSCT should be available only for EBV-HLH patients who relapse or show resistance to immunochemotherapy. In one retrospective study, a total of 14 patients with EBV-HLH underwent allogeneic SCT, including cord blood transplantation (CBT) in half of the cases [27]. The 10-year OS was 85.7 ± 9.4%, and the survival of CBT recipients was >65%. However, SCT with myeloablative conditioning is associated with high transplantation-related mortality [28]. Recently, reduced-intensity conditioning, which is less toxic and has a low incidence of long-term sequelae, has been used for primary HLH. Further studies are needed to determine the optimal SCT regimen and establish a comprehensive strategy for the cure of EBV-HLH.
Based on the hypothesis that the removal of EBV-infected B cells contribute to an improved prognosis of EBV-HLH, B-cell targeting monoclonal antibody rituximab may be another concept of treatment. Balamuth et al. [11] reported that adding rituximab to the HLH-2004 treatment protocol improved the efficacy of the treatment regimen. The EBV-HLH Rituximab Study Group [13] in the USA retrospectively examined 42 cases of EBV-HLH in whom rituximab was administered. In 18 cases (43%), clinical symptoms improved, and EBV and ferritin levels significantly decreased after the administration of rituximab; the survival rate was high in cases that showed such a decrease. Rituximab may be a possible therapeutic option during the course of therapy or at the end of initial therapy to remove proliferating EBV-infected B cells.
References
1.
2.
Ishii E, Ohga S, Imashuku S, Yasukawa M, Tsuda H, Miura I, et al. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007;86(1):58–65.CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree