Fig. 13.1
The catheterization method for intra-arterial infusion. (a, b) Catheterization into ECA near a tumor-feeding artery via STA (a) or a superior thyroid artery (b) with a straight catheter. (c) Catheterization into the tumor-feeding artery via a femoral artery using the Seldinger method. (d) Catheterization into the tumor-feeding artery via STA with a hook-shaped catheter. CCA common carotid artery, ICA internal carotid artery, ECA external carotid artery, LA lingual artery, FA facial artery, OA occipital artery, MA maxillary artery, STA superficial temporal artery
13.2 Conventional Intra-arterial Infusion via STA or a Superior Thyroid Artery (Catheterization into ECA)
Intra-arterial chemotherapy for head and neck cancer was first reported by Klopp et al. [1] and Sullivan et al. [2] in the 1950s. They reported insertion of a straight catheter into ECA via STA or a superior thyroid artery (Fig. 13.1a, b). The efficacy of treatment with intra-arterial chemotherapy has been reported [3–6].
Flow of the anticancer agents to the tumor bed is unstable with this method, because the tip of the catheter may be easily displaced near the tumor-feeding artery in ECA by neck extension. The tip of a straight catheter is located slightly peripheral to the tumor-feeding artery in ECA (Fig. 13.2a), and the anticancer agents can be administered to the tumor with a manual one-shot injection of high pressure (Fig. 13.2b). On the other hand, anticancer agents cannot be administered to the tumor when a syringe pump is used because of low pressure (Fig. 13.2c).
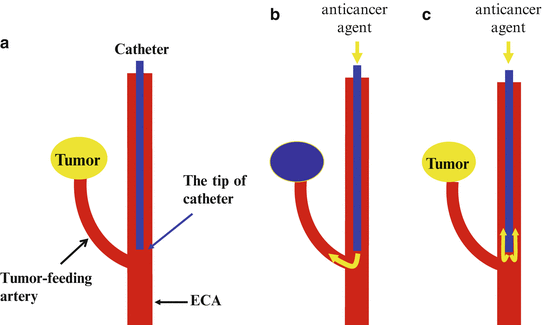

Fig. 13.2
(a) The tip of a straight catheter is located slightly peripheral to tumor-feeding artery. (b) The anticancer agents can be administered to the tumor with a manual one-shot injection of high pressure. (c) Anticancer agents cannot be administered to the tumor when a syringe pump is used because of low pressure
13.3 Superselective Intra-arterial Infusion via a Femoral Artery by the Seldinger Method
Lee et al. [7, 8] first reported superselective intra-arterial chemotherapy via a femoral artery using the Seldinger method (Fig. 13.3), and Robbins et al. [9–11] have developed a cisplatin (CDDP) delivery system in which extremely large amounts of anticancer agent with radiotherapy can be administered to patients with advanced head and neck cancer. Given the acronym “RADPLAT,” this consists of rapid superselective intra-arterial infusions combined with intravenous sodium thiosulfate for systemic CDDP neutralization. They conducted a phase I study designed to determine the maximum-tolerated dose of CDDP that could be administered in intra-arterial chemotherapy. The maximum-tolerated dose was 150 mg/m2/week for 4 weeks. In addition, this method has been found highly useful in the treatment of cervical lymph node metastasis [12, 13]. There have been many reports of RADPLAT for head and neck cancer [14–20], and RADPLAT has been widely accepted worldwide. However, Rasch et al. [21] reported that the local control rate and the survival rate of RADPLAT were no different from those of intravenous CRT in the Netherlands. Robbins questioned whether the result of this randomized trial was related to the technique used to deliver the intra-arterial infusions [22].
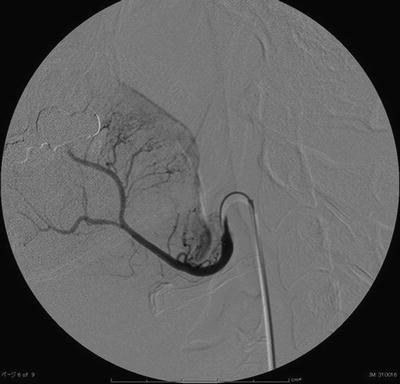

Fig. 13.3
Superselective intra-arterial infusion via a femoral artery by the Seldinger method. Tumor staining of the tongue from LA can be seen with use of contrast medium on digital subtraction angiography (DSA) (this figure was sponsored by Dr. Takamatsu S. at Fukui Prefectural Hospital)
Catheterization via a femoral artery using the Seldinger method sometimes causes serious problems, such as cerebral infarction and sudden death [7, 8]. There is an interesting report that institutions inexperienced in the use of RADPLAT had a higher rate of grades 4 and 5 toxicities related to cerebral infarction than experienced institutions did [17].
13.4 Superselective Intra-arterial Infusion via STA and an Occipital Artery (OA)
This method was developed to overcome the disadvantages associated with the techniques mentioned above. A hook-shaped catheter is inserted from STA in retrograde fashion into a tumor-feeding artery; the usefulness of this method in combination with radiotherapy has been reported [23–27].
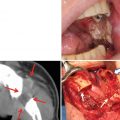
Before treatment, 3-dimensional computed tomography angiography (3D-CTA) of the carotid artery is necessary to identify the main tumor-feeding arteries and determine the morphology of the tumor-feeding artery originating from ECA (Fig. 13.4). Patients with severe calcification of the carotid artery or stenosis of the internal/external carotid artery are ineligible (Fig. 13.5a, b).
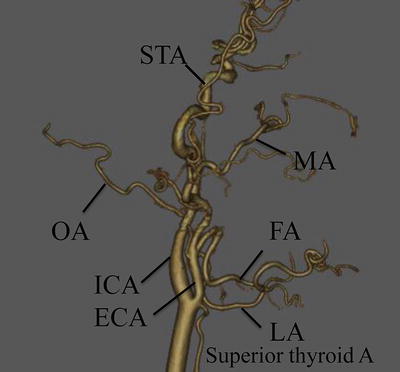
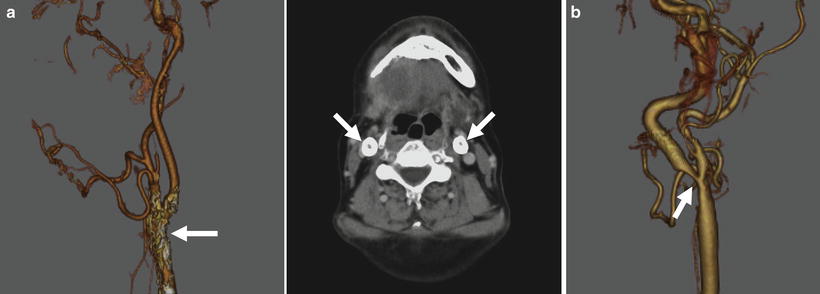

Fig. 13.4
Three-dimensional computed tomography angiography (3D-CTA) of the carotid artery

Fig. 13.5
Calcification of the external carotid artery (a: arrowhead) and stenosis of the internal carotid artery (b: arrowhead). Catheter insertion is contraindicated in these cases
Catheterization from STA is performed according to the method of Hattori, Fuwa and Tohnai [23–25] (HFT method) [28] (Figs. 13.6, 13.7, and 13.8). STA is exposed by a 30-mm skin incision in the preauricular region of the affected side (Fig. 13.7a, b). Indwelling needle is inserted into STA (Fig. 13.7c), and an inner needle is removed from an outer needle A 0.016-inch guidewire (GT wire, Terumo Corp., Tokyo, Japan) (Fig. 13.6a) is inserted into the common carotid artery through an outer needle (Fig. 13.7d). A vinyl hook-shaped catheter (NECK, 4 Fr in outer diameter, Medikit Corp., Tokyo, Japan) (Fig. 13.6b) is inserted into STA along the guidewire (Fig. 13.7e) and placed below the bifurcation of the target artery (Fig. 13.7f). The tip of the catheter is then superselectively inserted into the target artery by drawing it back under fluoroscopic guidance (Fig. 13.7g, h), and the position of the catheter is checked by injection of contrast medium and blue dye (Fig. 13.7i, j). When catheterization using a hook-shaped catheter is not stable, the guidewire exchange method is used to replace it with a polyurethane straight catheter (Fig. 13.6c) [29].
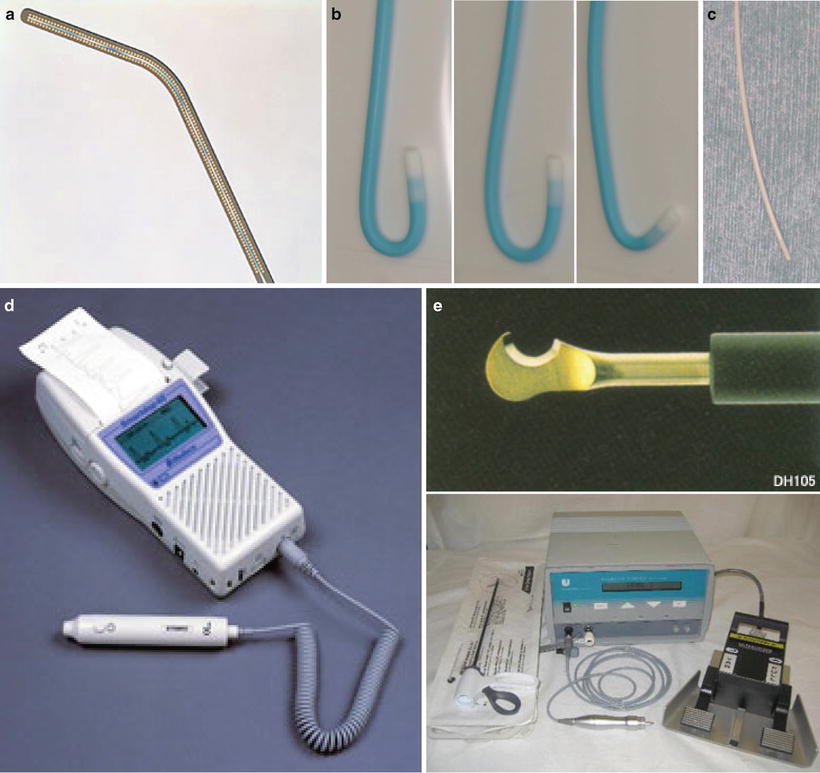
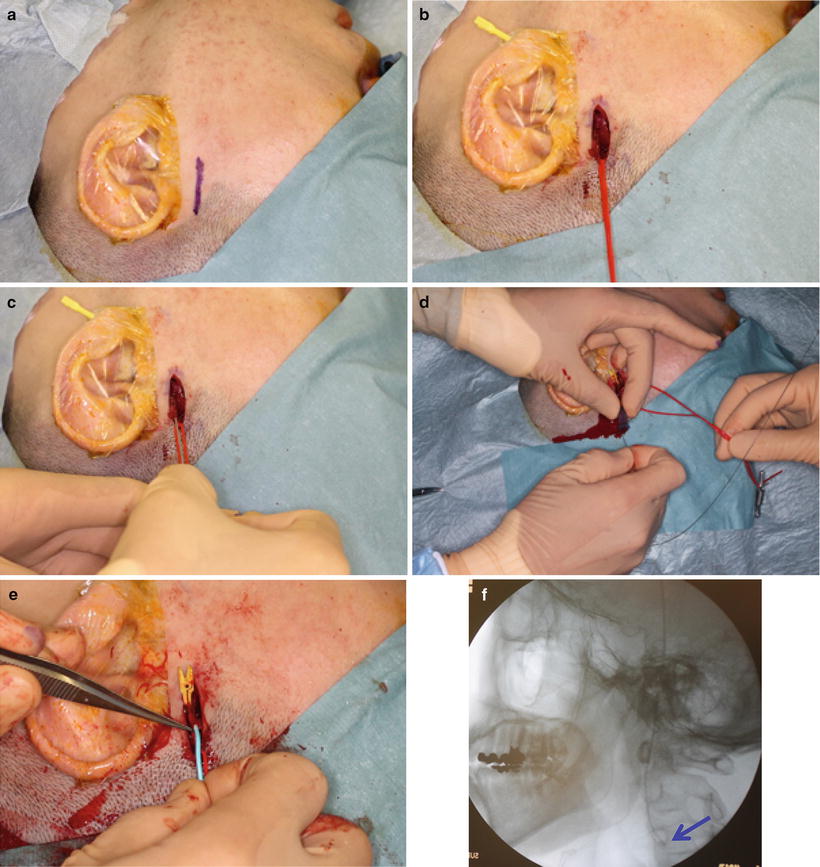
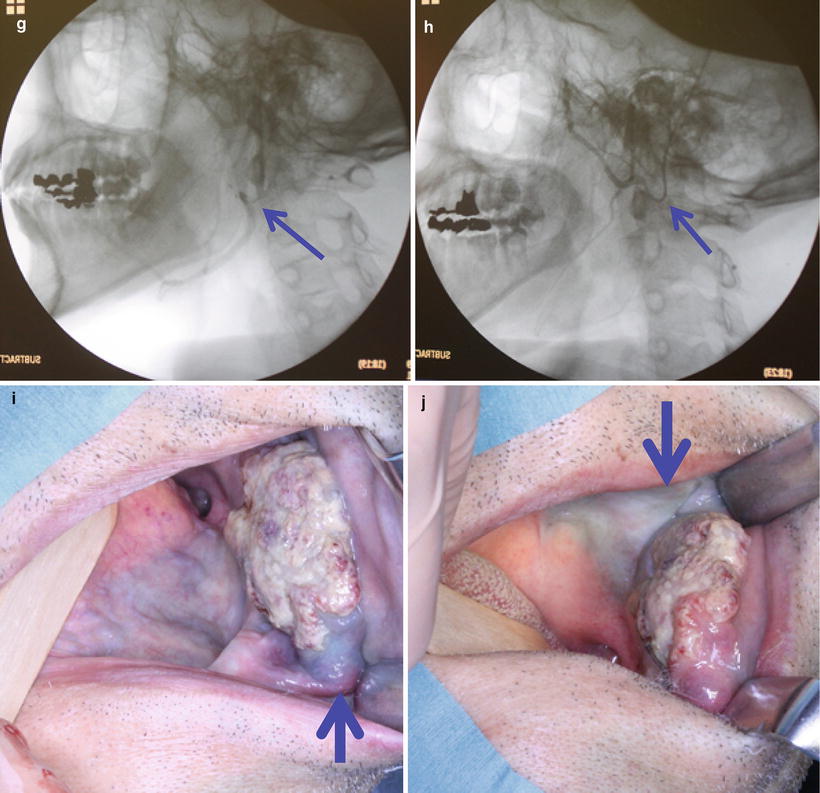
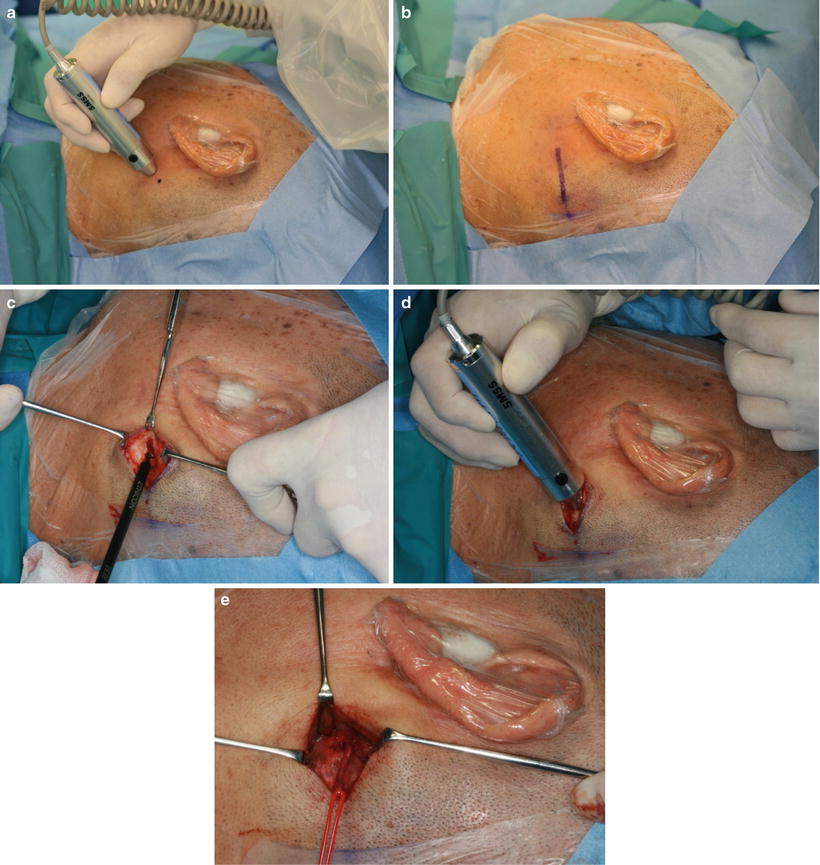

Fig. 13.6
(a) A 0.016-inch guidewire (Radifocus Guide Wire, Terumo Corp., Tokyo, Japan), (b) a hook-shaped catheter (Neck, Medikit Corp., Tokyo, Japan) (light, Neck 1G; middle, Neck 2G; right, Neck M), (c) a polyurethane straight catheter (Anthron P-U catheter, Toray Medical Co., Ltd., Tokyo, Japan), (d) ultrasonic blood flow detector (Smartdop 45, KDD Co, Ltd., Shiga, Japan), (e) electrosurgical diathermy (Harmonic scalpel, Johnson & Johnson K.K)


Fig. 13.7
STA is exposed by a 30-mm skin incision in the preauricular region (a, b). Indwelling needle is inserted into STA (c), and a guidewire is inserted into the common carotid artery (d). A hook-shaped catheter is inserted into STA along the guidewire (e) and placed below the bifurcation of the target artery (f, arrowhead). The tip of the catheter is then superselectively inserted into a target artery (g, facial artery, arrowhead) (h, maxillary artery, arrowhead). The position of the catheter is checked by injection of contrast medium and blue dye. Anterior and lower of the tumor is dyed from a facial artery (i, arrowhead), and posterior of the tumor is dyed from a maxillary artery (j, arrowhead).

Fig. 13.8
OA is identified posterior of the mastoid process by an ultrasonic blood flow detector (a). A 35-mm skin incision is made (b), and the sternocleidomastoid muscle and splenius capitis muscle are safely transected using the ultrasonic scalpel (c, d), and OA is exposed (e). Then, the catheter is superselectively inserted into the target artery under fluoroscopic guidance
When the tumor has two or more feeding arteries, catheters are inserted into the two arteries via STA and OA or bilaterally. Catheterization from OA is performed according to the method of Iwai et al. [30] (Fig. 13.8). OA is identified posterior of the mastoid process by an ultrasonic blood flow detector (Doppler ultrasound) (13.7d, and Fig. 13.8a). A 35-mm skin incision is made, and the sternocleidomastoid muscle and splenius capitis muscle are safely transected using the ultrasonic scalpel without OA injury (Fig. 13.7e, and 13.8b–e). Then, the catheter is superselectively inserted into the target artery under fluoroscopic guidance. The transected muscles, subcutaneous tissues, and skin are sutured, and the catheter is fixed to the skin around the mastoid process.
After catheterization, flow-check digital subtraction angiography (DSA) and angio-CT are performed in all cases (Fig. 13.9a–d). Angio-CT is helpful for detecting tumors by confirming the enhancement of the feeding area and for enabling the catheter to be placed at the appropriate position. Furthermore, weekly confirmation of the feeding artery by injection of a small amount of indigo carmine is important (Fig. 13.9e, f).
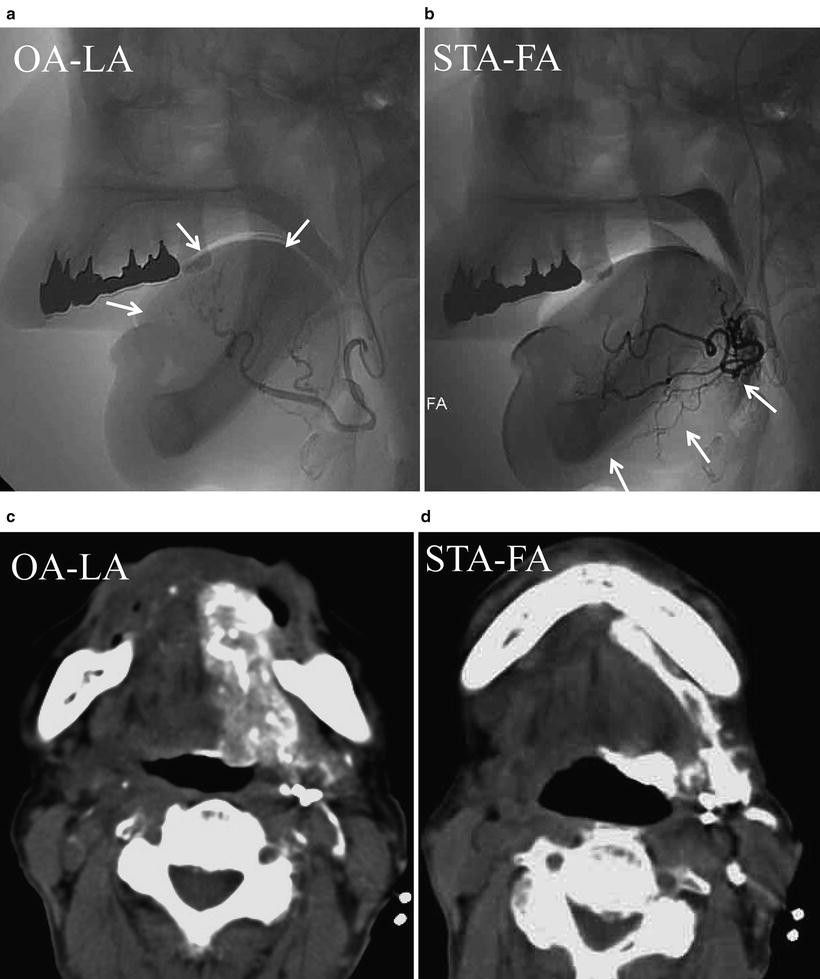
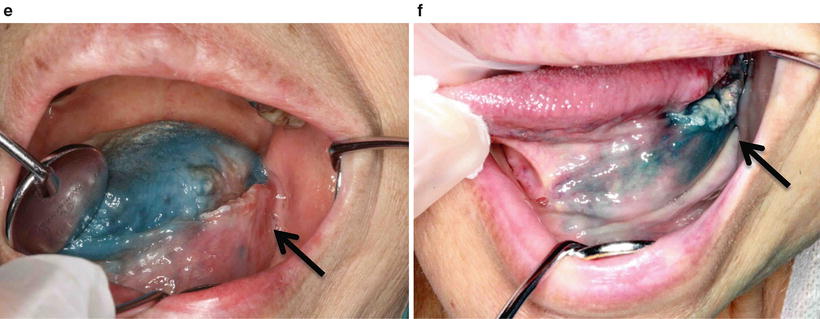


Fig. 13.9
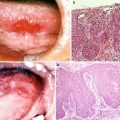
Squamous cell carcinoma of the tongue (T3N1M0). (a, b) DSA of retrograde superselective intra-arterial infusion. Two catheters are superselectively inserted into left LA via OA (OA-LA) (a) and left FA via STA (STA-FA) (b). Tumor stain is seen with the use of contrast medium on flow-check DSA (arrowhead). (c, d) Axial views of angio-CT. Angio-CT images show that tumor staining of the left tongue from left LA (c) and the left mouth floor from left FA (d) can be seen with the use of contrast medium. (e, f) The left side of the tongue tumor extends to the floor of the mouth. The perfusion area from left LA is not visible to the floor of the mouth (e, arrowhead), and the perfusion area of the floor of the mouth and the inside of the mandible is seen from left FA (f, arrowhead)
13.5 Radiotherapy
Radiotherapy is planned for all patients after appropriate immobilization using a thermoplastic mask and three-dimensional CT-based techniques. Conventional radiotherapy is performed with 4 or 6 MV at 2 Gy/fr/day. The irradiation field is changed according to lymph node status. In cases of N0 disease, the field contains the primary site and levels I–III of the neck on the ipsilateral side. The dose is delivered to 40 Gy/20fr. The portal is then reduced to only the primary site to spare the spinal cord. The total dose delivered to the primary tumor is 60 Gy/30fr. In cases of N1, N2a, and N2b diseases, the field contains the primary site and levels I–V of the neck on the ipsilateral side. The dose is delivered at 40 Gy/20fr. The portal is then reduced to the primary site and lymph node metastases. The dose for the spinal cord ranges from 40 Gy to 45 Gy. The total dose delivered to the primary tumor is 60 Gy/30fr and that to the metastatic lymph node sites is to 50 Gy/25fr. In cases of N2c disease, the field contains the primary site and levels I–V of the neck on bilateral sides. The dose is delivered at 40 Gy/20fr. The portal is then reduced to the primary site and lymph node metastases. The dose to the spinal cord ranges from 40 Gy to 45 Gy. The total dose delivered to the primary tumor is 60 Gy/30fr, and, if at all possible, the total dose delivered to the metastatic lymph node sites is 50 Gy/25fr.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree