Chapter 9
Chemoprevention
IF YOU’RE CONSIDERING OPTIONS to reduce your cancer risk, you have two choices: chemoprevention, as described in this chapter, and surgery (chapters 10 and 12). Taking chemoprevention medications doesn’t guarantee you’ll never develop cancer, but it can decrease your risk. If you still have your breasts and ovaries, you’ll need to maintain surveillance to find any early cancers. Some women take chemoprevention drugs and then later further reduce their risk with surgery.
Chemoprevention isn’t for everyone. You may welcome its noninvasive risk-reducing potential or may decide the possible side effects outweigh the benefits, depending on your overall health and tolerance for risk. Some studies of these medications haven’t specifically focused on high-risk women; fewer have focused specifically on BRCA mutation carriers. A healthcare team with expertise in managing high-risk patients can give you a clear sense of how various chemoprevention drugs might affect your risk.
Risk-Reducing Medications for Breast Cancer
Tamoxifen and raloxifene are FDA-approved medications that reduce the risk of developing breast cancer in high-risk women. Both are selective estrogen receptor modulators (SERMs), drugs that block estrogen. Another SERM, fareston, is used to treat postmenopausal metastatic breast cancer. It isn’t approved for chemoprevention.
Tamoxifen
Well-studied tamoxifen (marketed as Nolvadex tablets and Soltamox liquid) has been standard treatment for ER+ breast cancers since 1977. The drug has an impressive track record for treating breast cancer and lowering recurrence. Study data show tamoxifen:
• shrinks large ER+ tumors before surgery.
• cuts in half the risk of a new cancer in the opposite breast.
• lowers recurrence 40 to 50 percent in postmenopausal women and 30 to 50 percent in premenopausal women.
• reduces risk of recurring DCIS or invasive breast cancer by 50 percent when used after lumpectomy and radiation treatment for DCIS.
Researchers have studied the benefit of tamoxifen in different populations of women, with different results for each group. In a study of women with BRCA-related cancer in one breast, those who took tamoxifen demonstrated a significantly reduced risk in the other breast.1 The medication appeared to work for both BRCA1 and BRCA2 mutation carriers. The research on tamoxifen for previvors shows different results. A large U.S. study showed that tamoxifen substantially reduced the chance for breast cancer in high-risk women.2 Most participants didn’t have BRCA mutations. Among the nineteen women with BRCA mutations, only those with a mutation in BRCA2 benefited. The medication didn’t seem to help women who had BRCA1 mutations. A second study in the United Kingdom reviewed a similar group of high-risk women who took tamoxifen for eight years and were then followed for twenty years.3 Participants weren’t tested to determine whether any of them had BRCA mutations. This study showed a reduced risk for ER+ breast cancers, but not for ER– breast cancers.
Based on this limited research, most experts believe tamoxifen effectively decreases risk for breast cancer in survivors and previvors, especially those who have BRCA2 mutations. Not all experts agree that it is equally effective for BRCA1 mutation carriers, who tend to develop ER– cancers. If you’re considering tamoxifen for chemoprevention, discuss the benefits and limitations with your oncologist.
Raloxifene
Marketed as Evista, raloxifene functions much like tamoxifen, with less risk for uterine cancers. Raloxifene was originally developed to prevent and treat osteoporosis, but doctors noticed that women who took it for bone loss had a reduced risk of invasive breast cancer. The Study of Tamoxifen and Raloxifene (STAR) trial in 1999 showed that both medications reduced high-risk postmenopausal women’s odds (as defined by the Gail Model) of developing invasive breast cancer by about half during the five years they took it and for ten years following. Based on these results, raloxifene is FDA approved for breast cancer prevention in postmenopausal women. No studies have yet explored whether it prevents breast cancer specifically in BRCA mutation carriers. Raloxifene also works against DCIS, which can develop into breast cancer, and LCIS. Compared to tamoxifen, the benefits of raloxifene decline more quickly once women stop taking it.
Side Effects of SERMs
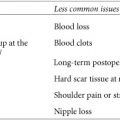
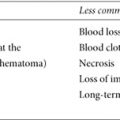
Tamoxifen and raloxifene are reliable and effective multitasking medications. Aside from potentially preventing breast cancer, they improve bone density. Both, however, have side effects. Taking either medication may cause hot flashes, vaginal dryness, mood changes, and other menopause-like symptoms, which may be mild and well tolerated by some women; others need medication to address their symptoms. SERMs can also cause vaginal bleeding or discharge, headaches, nausea, leg cramps, rashes, and other serious side effects.
Tamoxifen slightly increases a woman’s risk for endometrial cancer—the risk is still relatively low, about two chances in 1,000 per year.4 Raloxifene has less risk of uterine cancer. Unlike tamoxifen, it doesn’t have an estrogen-like effect on the uterus. These medications also increase the chance of stroke and blood clots, particularly in those who smoke or have clotting disorders. Neither drug is appropriate for women with a Factor V Leiden mutation, those who have (or have had) blood clots, or those taking estrogen replacement or certain cholesterol-reducing drugs, including Locholest and Questran.
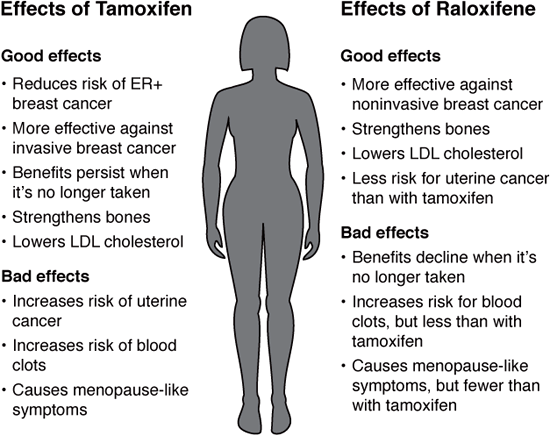
Comparing tamoxifen and raloxifene
Aromatase Inhibitors
Aromatase inhibitors (AIs), including exemestane (Aromasin), letrozole (Femara), and anastrozole (Arimidex) treat postmenopausal ER+ and PR+ breast cancers. For survivors, AIs work more effectively than tamoxifen against advanced breast cancer; following five years of tamoxifen with five years of an AI continues to reduce your risk of recurrence. Health experts are hoping AIs prevent breast cancer in postmenopausal previvors as successfully as they treat breast cancers in women with sporadic, hormone-positive breast cancers.
You may wonder why postmenopausal women need to be concerned about estrogen. After menopause, even though your ovaries don’t produce much estrogen, your body converts fat and other hormones into estrogen to a lesser degree than before menopause; it’s still enough to stimulate estrogen receptors on the breast. This is one reason why being overweight contributes to breast cancer risk. While tamoxifen and raloxifene block estrogen from breast cancer receptors, AIs reduce up to 95 percent of postmenopausal estrogen. AIs don’t affect the amount of estrogen made by the ovaries; that’s why they’re not effective for premenopausal women. Unlike SERMs, AIs don’t improve bone density. In fact, they may accelerate postmenopausal bone loss. Unlike tamoxifen and raloxifene, AIs are less likely to cause serious blood clots and don’t cause uterine cancers—they can cause hot flashes, vaginal dryness, headaches, muscle or joint pain, and other short-term side effects similar to those caused by tamoxifen.
AIs aren’t FDA approved to prevent breast cancer. They could be prescribed as a preventive measure in the future if research shows they effectively reduce risk. AIs haven’t been studied as much as SERMs, so experts don’t yet know about their long-term effects or risks. The Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial found Arimidex reduced the risk of a new cancer in the opposite breast of survivors by 58 percent.5 The study didn’t specifically include women with BRCA mutations, so its preventive benefits in mutation carriers remains uncertain. Clinical studies are under way to determine whether AIs reduce postmenopausal breast cancer in high-risk women, including BRCA carriers. You can track the status of these trials at clinicaltrials.gov.
MY STORY: For Me, Surveillance Alone Isn’t Enough
When I tested positive for a BRCA1 mutation at age 27, I wasn’t ready for prophylactic surgery to reduce my risk, so my oncologist suggested I take tamoxifen for five years. I knew it was controversial for prevention, but I researched it carefully. If my body didn’t react well to surgical menopause or I had complications from mastectomy, that could affect my life forever. Taking a pill didn’t feel that way, and I would be doing something more than just surveillance. I decided to try it for six months; if I didn’t like how I felt or couldn’t handle the side effects, I would stop taking it. I was comfortable with my decision, and I had an easy out if I didn’t want to continue. As it turned out, I tolerated tamoxifen very well, maybe because I was so young when I started taking it or because I was proactive with my diet and lifestyle. Maybe I was just lucky. I took a risk with tamoxifen; it was the best decision I could have made.
—CARI
Alternatives under Study
Nonsteroidal Anti-inflammatory Drugs
Because inflammation contributes to many diseases, including cancers, some scientists speculate that aspirin and other anti-inflammatory medications might reduce cancer risk. Research to analyze the effects of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) on breast cancer risk has been inconclusive, and studies haven’t specifically focused on high-risk individuals. NSAIDs include several common over-the-counter painkillers, including ibuprofen (Advil, Motrin, and others) and naproxen sodium (Aleve).
In the Women’s Health Initiative, a national fifteen-year study of cardiovascular disease, cancer, and osteoporosis in postmenopausal women, individuals over age 50 who used aspirin regularly had a 21 percent decreased chance of developing breast cancer.6 Regular use of ibuprofen was associated with a 49 percent reduction in breast cancer risk, even in women who had first-degree female relatives (mother, sister, or daughter) with breast cancer. The study was observational only: women who regularly took NSAIDs were less likely to develop breast cancer, but researchers can’t be certain if the NSAIDs or some other factor produced the benefit. Nor did the study specifically focus on high-risk women. The Nurses’ Health Study, a large, long-term research effort following 76,821 nurses, showed that aspirin or other NSAIDs taken at least twice weekly don’t reduce premenopausal breast cancer risk.7
Certain NSAIDs increase the potential for death from heart disease. A clinical trial studying whether Celebrex could lessen the risk for colon polyps was discontinued when participants who took the medication suffered more heart disease and related deaths compared to participants who took a placebo. Even though the risk of heart disease-related death was low—about 3 percent of people taking the highest dose and 2 percent risk in those who were taking a lower dose—in this particular study, the risks of Celebrex outweighed the benefits.8 Other clinical trials are exploring whether nonsteroidal anti-inflammatory agents decrease breast cancer in high-risk women, including some studies of BRCA mutation carriers. Some medications in this category, including Celebrex and others, increase the risk of heart attack and stroke and shouldn’t be used by anyone at high risk for those conditions.
Fenretinide
Research suggests that fenretinide, a medication related to vitamin A, might prevent new breast tumors in premenopausal women who have been previously diagnosed. Over fifteen years, 1,739 patients previously diagnosed with DCIS or stage 1 breast cancer who weren’t treated with chemotherapy took fenretinide and had 17 percent lower incidence of a second breast cancer diagnosis than women who didn’t take the medication. Fenretinide was effective primarily in premenopausal women; the younger the woman, the more fenretinide reduced risk: 35 percent less in women under age 50, and 50 percent less in women under age 40.9 The drug’s protective benefit continued even after women stopped taking it. Impressive results, yet the medication actually increased risk for a second breast cancer in women age 55 and older. The study didn’t include previvors, so researchers can’t conclude that fenretinide prevents breast cancer in these women.
Statins
Millions of people around the world take statins to reduce cholesterol and lower their risk of heart attack. One observational, retrospective study found that women who took statins regularly were 51 percent less likely to develop breast cancer.10 Researchers can’t be certain statins get the credit—in other studies involving women of average risk, these drugs haven’t shown a protective effect. Studies are under way to determine whether statins reduce risk for high-risk women.
Deslorelin
Removing the ovaries lowers breast cancer risk, particularly in women with BRCA mutations. Deslorelin acetate prevents ovaries from producing estrogen, fueling speculation that it may also protect against breast cancer. Unlike surgical removal of the ovaries, deslorelin’s effect is reversible: once a woman stops taking it, her ovaries begin making estrogen again. One preliminary study of deslorelin in premenopausal women with BRCA mutations showed it decreased breast density, which is linked to lower breast cancer risk and improved detection of early stage tumors by mammography. This research is encouraging; more study is needed to determine whether deslorelin lowers breast cancer risk.
Bisphosphonates
Data from the Women’s Health Initiative show that women who took oral bisphosphonates, bone-building medications used to treat osteoporosis, were 30 to 40 percent less likely to develop invasive hormone receptor-positive breast cancer than women who didn’t take the medication.11 Surprisingly, women who used either Fosamax, Actonel, or Boniva were 58 percent more likely to develop DCIS. Researchers aren’t sure why or how this occurs. They theorize that the medication somehow delays DCIS from evolving into invasive cancer. That would explain why there were fewer invasive cancers and more DCIS during the eight years of the study. An Israeli study found similar results, showing that women who took bisphosphonates for at least one year were 39 percent less likely to develop breast cancer than women who never took the drugs.12 Whether these drugs can be used proactively remains to be seen and requires clinical trials. Bisphosphonates are associated with an increased risk of femur (thigh bone) fractures and esophageal cancer; the FDA now requires this information to be included in the safety labels of these medications.
Metformin
The most common medication used to treat type 2 diabetes may also inhibit breast cancer cell growth. Increasingly, evidence is mounting that metformin not only reduces blood sugar and insulin levels, it may improve breast cancer outcomes or even decrease risk for new breast tumors. In one study of 22,621 European women with type 2 diabetes, those who took metformin for five years or more had about 60 percent less chance of developing breast cancer compared to those who used other medications.13 Metformin may act on both ER+ and triple-negative breast cancers, raising the possibility that it could be equally effective for BRCA1 and BRCA2 mutation carriers.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree