CHEMISTRY
Vasopressin and oxytocin are the only hormones known to be secreted in significant amounts by the neurohypophysis. As first shown by du Vigneaud and coworkers,4 the two hormones have similar structures (Fig. 25-2), being nonapeptides that contain six-membered disulfide rings and the same amino acid residues in seven of the nine positions. They are stored in neurosecretory granules as insoluble complexes with specific carrier proteins known as neurophysins.5 The neurophysins associated with vasopressin and oxytocin also have similar structures, each having ˜100 amino-acid residues with extensive areas of homology. Binding of the hormones to their neurophysins has a pH optima (5.2–5.8) and dissociation constant (Kd) (˜5 × 105) that favor association in neurosecretory granules but ensures almost complete dissociation in plasma and other body fluids.
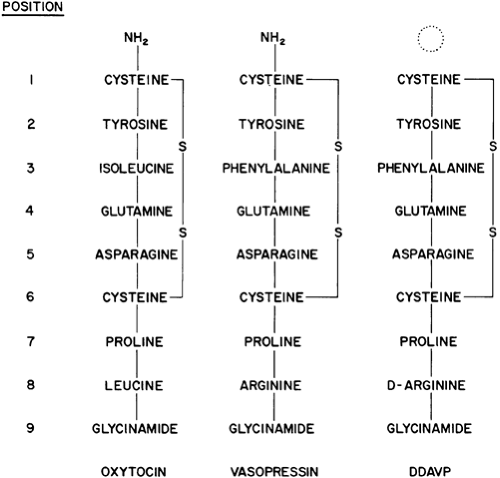 FIGURE 25-2. Structure of oxytocin, vasopressin, and 1-deamino-(8-D-arginine)-vasopressin (DDAVP). (S, sulfur.) |
BIOSYNTHESIS AND RELEASE OF VASOPRESSIN AND OXYTOCIN
Vasopressin and oxytocin are synthesized through protein precursors encoded by single-copy genes that are located near each other on chromosome 20 in humans6,7,8 and 9 (Fig. 25-3). The gene for the vasopressin precursor, known as propressophysin or vasopressin neurophysin II, is ˜2-kb long. It contains three exons that encode, respectively, (a) a signal peptide, vasopressin, and the variable amino-terminal end of neurophysin; (b) the highly conserved middle portion of neurophysin; and (c) the variable, carboxy-terminal end of neurophysin and copeptin, a glycosy-lated peptide of unknown function. The gene for the oxytocin precursor is similar except that exon C is shorter and codes only
for the variable carboxy terminus of neurophysin and a single histidine residue. The copeptin moiety is absent.
for the variable carboxy terminus of neurophysin and a single histidine residue. The copeptin moiety is absent.
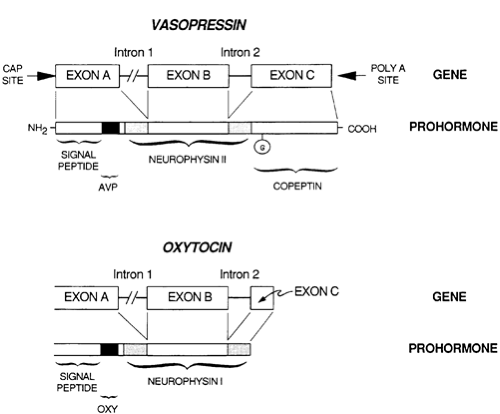 FIGURE 25-3. Structure of the vasopressin and oxytocin prohormones and the genes that encode them. (AVP, arginine vasopressin; G, glycosylation.) |
In humans and other mammals, the vasopressin and oxytocin genes are expressed in different magnocellular neurons. After transcription and translation in cell bodies within the supraoptic and paraventricular nuclei, the preprohormones are translocated into the endoplasmic reticulum, where the signal peptide is removed and the prohormones fold and self-associate before moving through the Golgi apparatus and on into the neurosecretory granules. There they are transported down the axons and cleaved into the intact hormone, neurophysin, and, in the case of vasopressin, copeptin. This process is critically dependent on correct folding and self-association of the prohormone in the endoplasmic reticulum, because genetic mutations predicted to alter amino acids important for correct folding severely disrupt transport and destroy the neurons, producing the autosomal dominant form of familial neurohypophyseal diabetes insipidus.
Release of the hormones and their associated neurophysins occurs via a calcium-dependent exocytotic process similar to that described for other neurosecretory systems.10 An electrical impulse propagated along the neuron depolarizes the cell membrane, causing an influx of calcium, fusion of secretory granules with the outer cell membranes, and extrusion of their contents.
REGULATION OF VASOPRESSIN SECRETION
OSMOTIC REGULATION
The secretion of vasopressin is influenced by a number of variables.11,12,13,14 and 15 The most important under physiologic conditions is the effective osmotic pressure of plasma. This influence is mediated by specialized cells called osmoreceptors. These osmoreceptors appear to be concentrated in the anterolateral hypothalamus,16,17 and 18 in an area that is near, but separate from, the supraoptic nuclei (see Fig. 25-1). This area is supplied with blood by small perforating branches of the anterior cerebral or communicating arteries.1
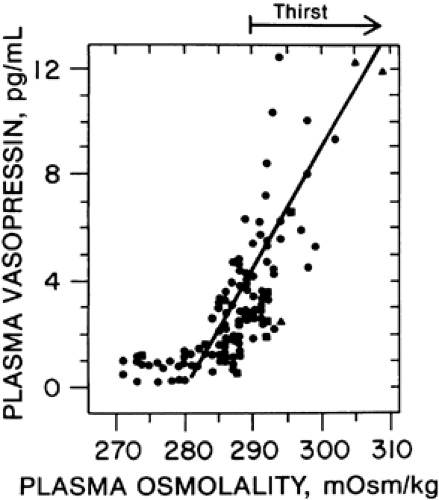
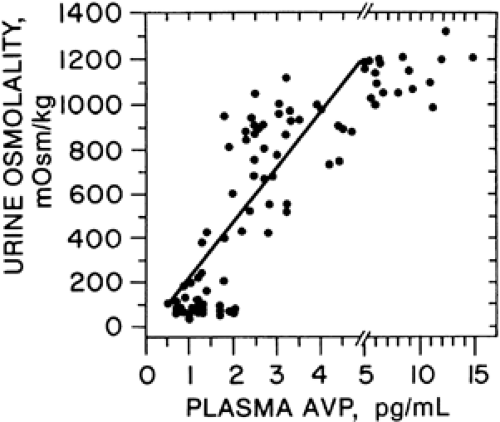
The basic structure, modus operandi, and organization of the individual osmoreceptors have not yet been determined. However, the system as a whole functions like a discontinuous or set-point receptor (Fig. 25-4). Thus, at plasma osmolalities below a certain minimum or threshold level, plasma vasopressin is suppressed to low or undetectable concentrations. Above this set-point, plasma vasopressin rises steeply in direct proportion to plasma osmolality. The slope of the relationship indicates that a change in plasma osmolality of only 1% alters plasma vasopressin by an average of 1 pg/mL, an amount sufficient to significantly affect the urinary concentration and flow (Fig. 25-5).
The sensitivity and “set” of the osmoregulatory system varies considerably among healthy adults. The interindividual differences in sensitivity are large (up to 10-fold), are constant over long periods of time, and appear to be genetically determined.19 However, they can be altered slightly by a variety of pharmacologic or pathologic influences.20 The interindividual differences in setpoint are not as large (275–290 mOsm/kg) or as constant over time but also appear to have a significant genetic component.19 They are more subject to alteration by a variety of physiologic factors, including posture, pregnancy, and the phase of the menstrual cycle,20,21 all of which lower the osmotic threshold or setpoint.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree