Abstract
Infective, granulomatous and benign histiocytic disorders are only rarely an indication for bone marrow (BM) examination and therefore often represent unexpected or incidental findings. Yet since they are frequently linked to a variety of life-threatening underlying conditions, diagnosing such disorders in the BM is almost always significant for the affected patient. This chapter summarizes and illustrates the most common disorders of this type in the BM with a special emphasis on diagnostic and differential diagnostic clues.
Introduction
Infective, granulomatous and benign histiocytic disorders are only rarely an indication for bone marrow (BM) examination and therefore often represent unexpected or incidental findings. Yet since they are frequently linked to a variety of life-threatening underlying conditions, diagnosing such disorders in the BM is almost always significant for the affected patient. This chapter summarizes and illustrates the most common disorders of this type in the BM with a special emphasis on diagnostic and differential diagnostic clues.
Bone Marrow Findings in Infective Disorders
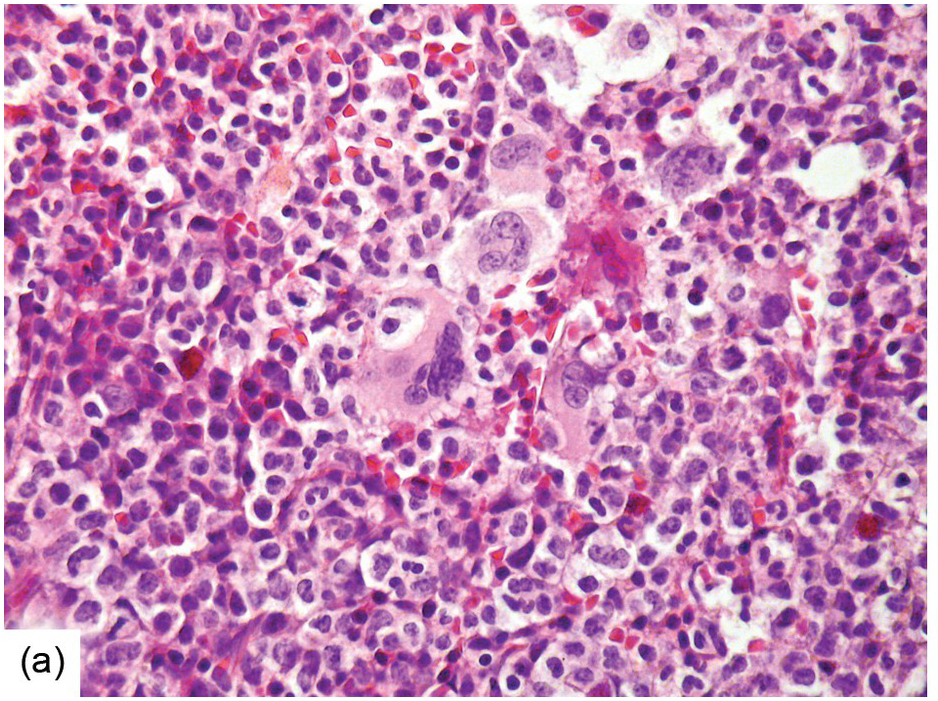
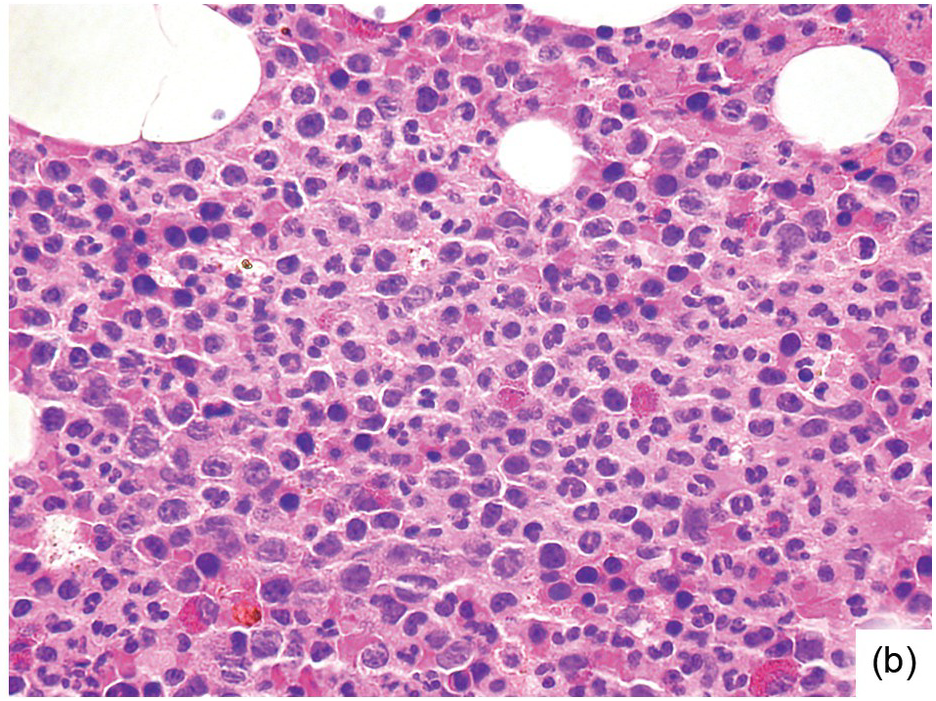
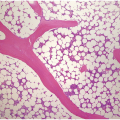
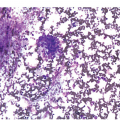
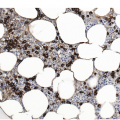
Being the primary source of cellular components of both inborn and acquired immunity, the BM is almost always involved in infections, particularly in systemic types. Bone marrow findings in infections are generally non-specific and mainly reflect the effects of cytokines (especially IL6) with a general increase in cellularity, left shift of megakaryopoiesis and myelopoiesis (Figure 6.1a) up to a leukaemoid reaction with proliferation of myelocytes and promyelocytes, and monocytic precursors that may be prominent enough to raise concerns of acute myeloid or chronic myelomonocytic leukaemia (Figure 6.1b).
(a) Highly hypercellular bone marrow with myeloproliferative-like features and emperipolesis of nucleated cells in the megakaryocytes in a patient suffering from subacute septic pleuritis.
(b) Leukaemoid reaction reminiscent of chronic neutrophilic leukaemia in a patient suffering from subacute urocystitis.
Interstitial oedema and small haemorrhages associated with arteriolo-capillary congestion and coagulation, and occasionally stellate fibrin deposits between the haematopoietic cells, are regularly observable in sepsis [1], which only exceptionally applies to BM biopsies but is regularly seen in post-mortem BM examinations in the respective setting. In more severe conditions, larger areas of haematopoietic hypoplasia, haemophagocytosis (see later) and ischaemic type necrosis, secondary to arteriolar thrombosis, may be seen [1]. These foci of necrosis should be distinguished from tumour cell necrosis observable in acute leukaemias and from caseous necrosis in tuberculosis by means of careful morphological examination of the surrounding bony trabeculae (usually vital in both later conditions), of the vessels (often thrombosed in septic conditions), high-power examination for ghost cells in tumour cell necrosis, and can be supported by properly applied ancillary techniques such as immunohistochemistry (IHC) for blast-markers and acid-fast stains.
Suppurative type lesions are characteristic of acute osteomyelitis but not of sepsis [1]. The topic of osteomyelitis will not be addressed here and reference should be made to some excellent review papers [2, 3].
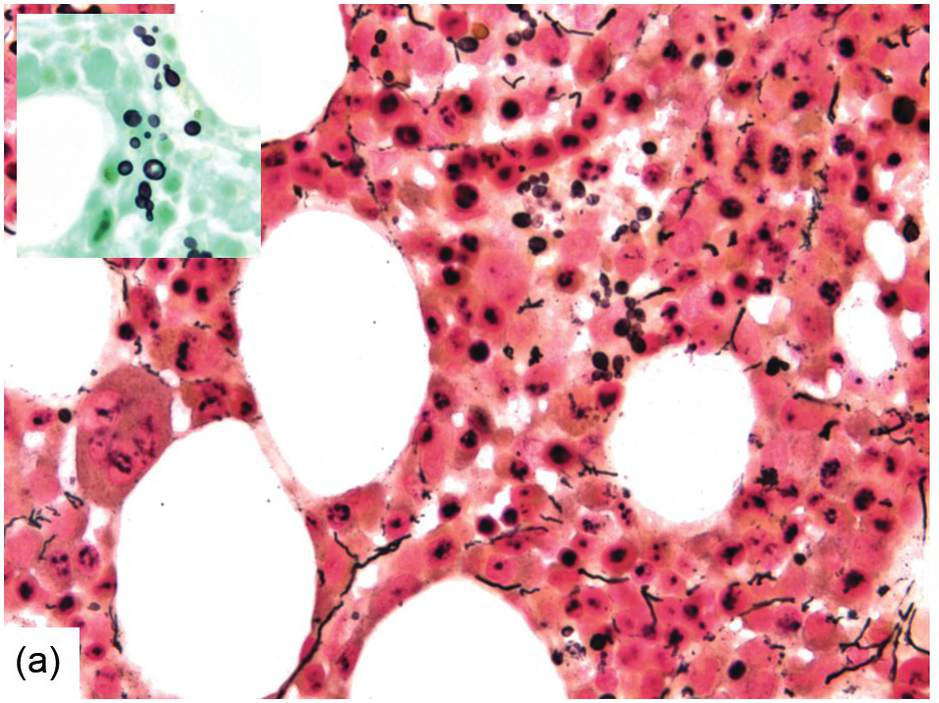
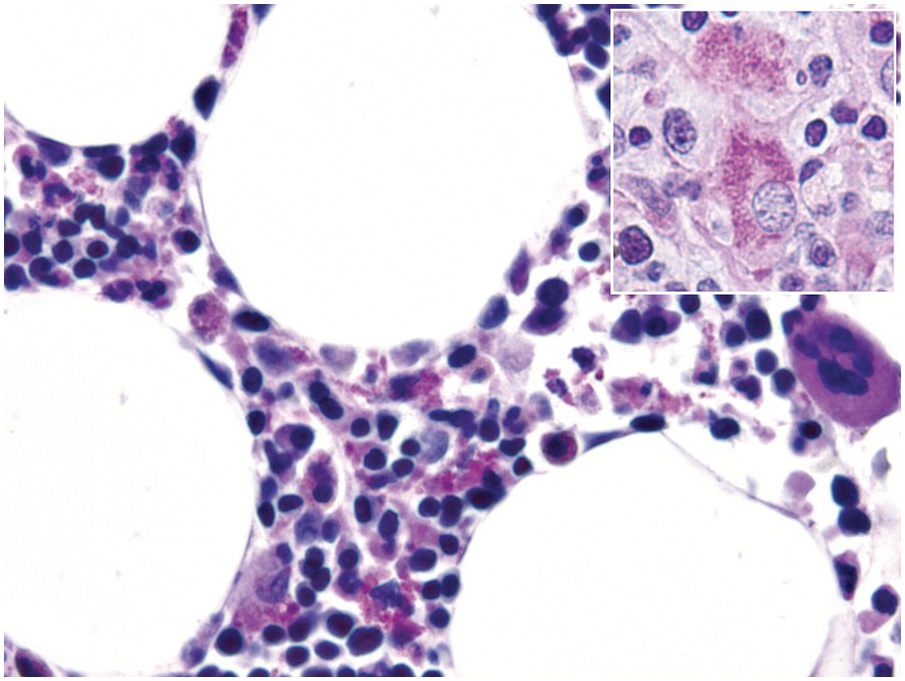
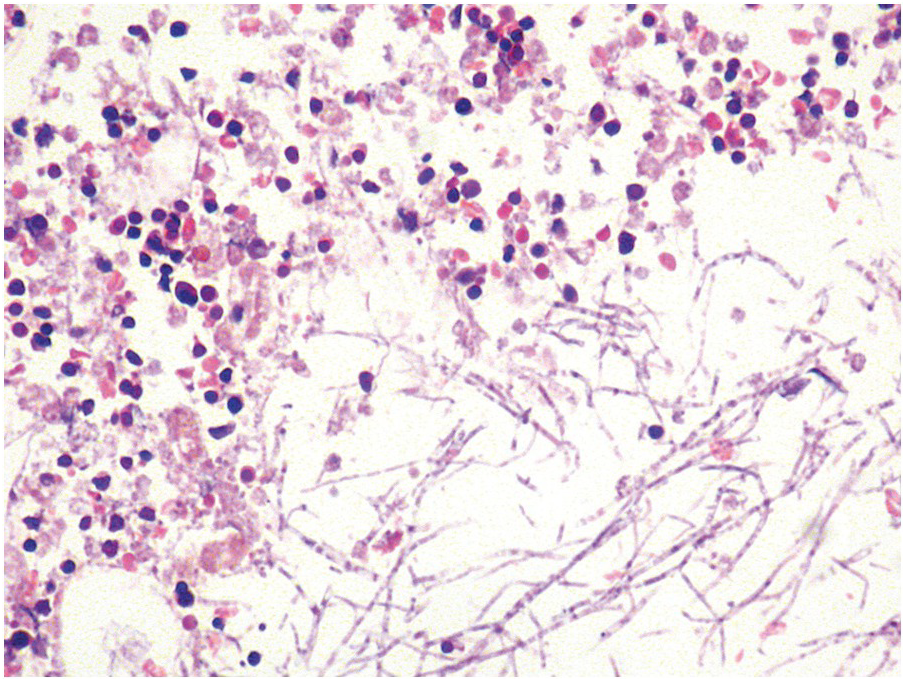
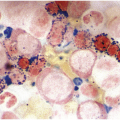
Occasionally BM changes in infectious diseases are more specific and may strongly suggest, or even be diagnostic of, a particular aetiologic agent (e.g. visible fungus or pathognomonic virus-cytopathic effects, Figure 6.2a,b; Table 6.1). From the perspective of BM examination, the work-up for systemic infections involves morphologic analysis, application of special stains, IHC or in situ hybridization as well as molecular studies to identify genetic material of the respective species, which should be followed by an integrative synopsis taking into consideration all results [4]. The importance of an integrative approach starting with the peripheral blood findings cannot be overestimated, bearing in mind that while most acute bacterial infections are accompanied by neutrophilic leukocytosis, in cases of pertussis and respiratory syncytial virus infection characteristic cleaved lymphocyte nuclei can be detected [5], and some chronic bacterial infections may be accompanied by monocytosis. Marked CD8-skewed lymphocytosis is characteristic of virus infections. Some infectious agents such as Plasmodium spp. or fungi may even be identifiable in the peripheral blood film [4]. Non-specific secondary/compensatory BM changes related to peripheral cell consumption in infections will not be further addressed here.
(a) Histoplasma capsulatum spreading to the bone marrow. Note the visible fungi in the Gömöri silver impregnation regularly performed on that biopsy; unless a careful histopathologic analysis is performed the fungi may be easily overlooked assuming impregnated nuclei. Inset: Grocott silver impregnation of the same case.
(b) Characteristic large erythroblast with nuclear inclusion in a case of complicated parvovirus B19-infection of the bone marrow. Inset: immunohistochemical proof of viral antigens in the respective cells.
Table 6.1 Histopathologic pattern-based diagnostic and differential diagnostic considerations in infective bone marrow diseases.
| Bone marrow patterns in infections | Probable/possible diagnosis | Differential diagnoses | Ancillary techniques |
|---|---|---|---|
| Coagulation, oedema, haemorrhage, necrosis | Septic changes | Tuberculosis Coagulopathy, Morbus embolicus Systemic lupus erythematosus | Ziehl–Neelsen stain Imaging studies Serology & Sequencing |
| Hypercellularity, haematopoietic left-shift | Subacute and chronic infections, paraneoplastic conditions (hepatic and renal neoplasms, mesothelioma) | Myeloid neoplasias | Immunohistochemistry History of drug exposure Flow cytometry immunophenotyping (e.g. Ogata score) Serology & Sequencing Cyto- and molecular genetics |
| Myelodysplastic changes | HIV-associated myelopathy, drug-induced changes, anaemia of chronic disease | ||
| Lymphocytosis – increased haematogones – CD4 skewed – CD8 skewed | Non-specific, common in children More common in drug reactions More common in viral diseases | B-acute lymphoblastic leukaemia T-cell lymphomas | Immunohistochemistry History of drug exposure FACS (e.g. clonality studies) Cyto- and molecular genetics |
| Suppuration | Osteomyelitis, cat-scratch disease | Exudative neutrophilia in osteoporotic microfractures | Brown-Brenn-, Gram-, Grocott- and Warthin–Starry stains Imaging studies Serology & Sequencing |
| Visible microorganisms | Fungus or parasite spread | Artefact or contamination | Serial sections Histo- and immunohistochemistry Serology & Sequencing |
| Visible virus-cytopathic effects | CMV-, HHV6- or parvovirus B19 infection | Artefact | Immunohistochemistry Serology & Sequencing |
Viral Infections
With a few exceptions, work-up for suspected viral infections rarely includes BM examination, thus morphological hints observable in a BM biopsy or aspirate suggesting such an infection are often unexpected findings.
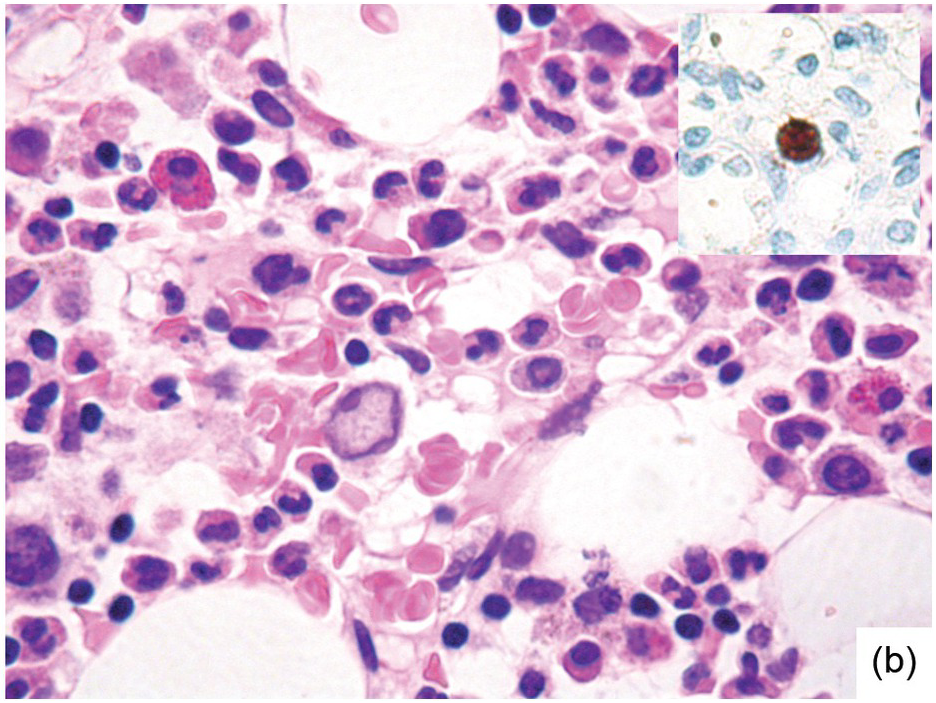
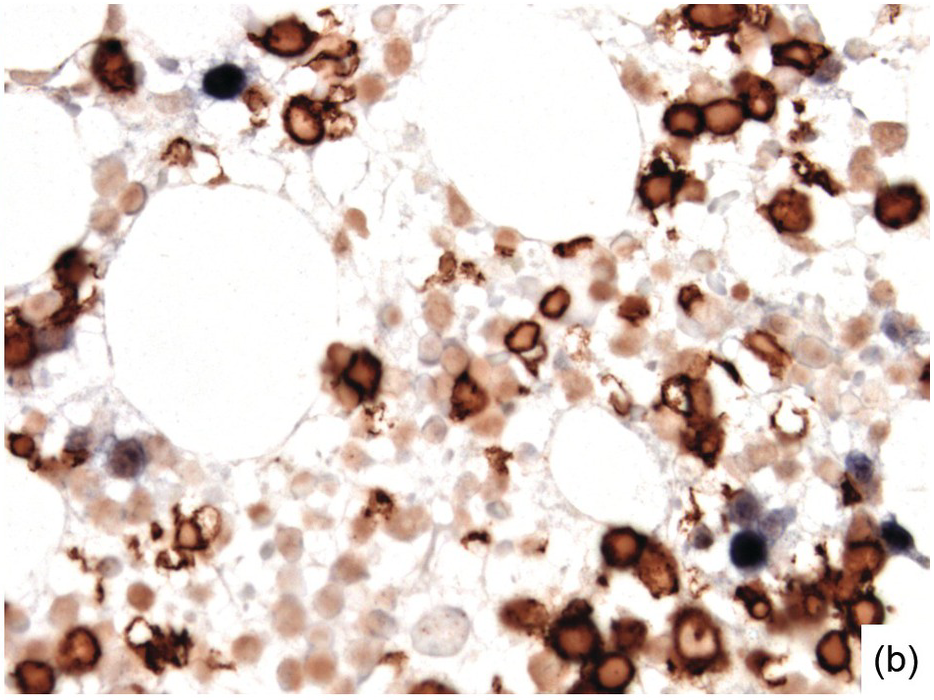
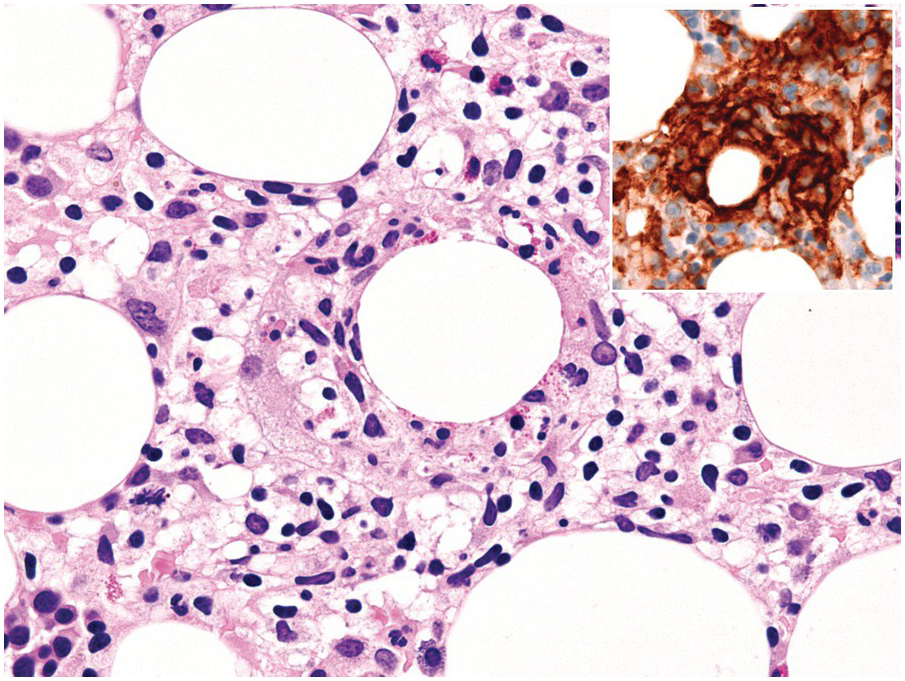
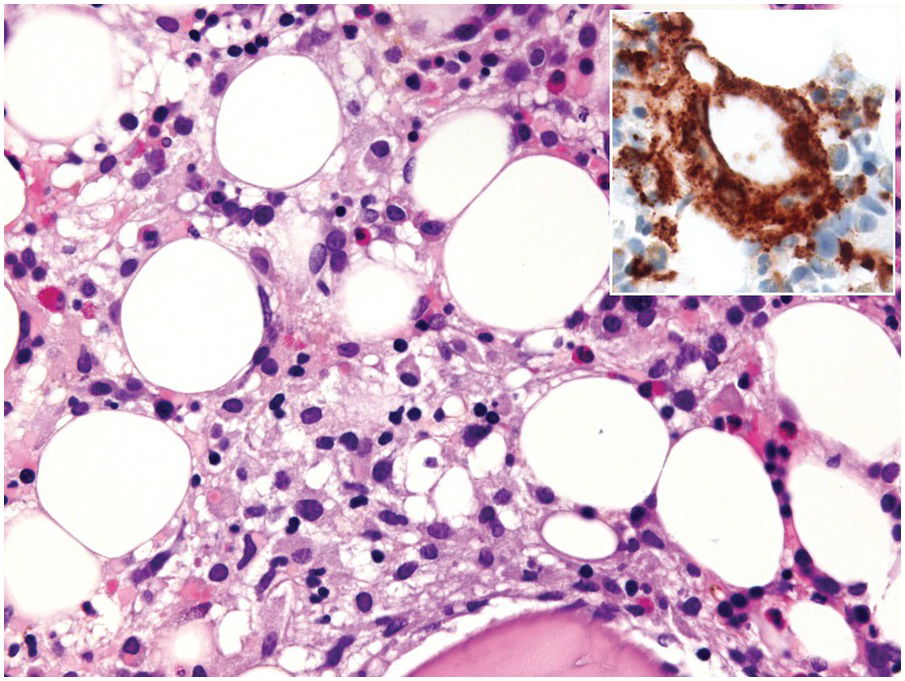
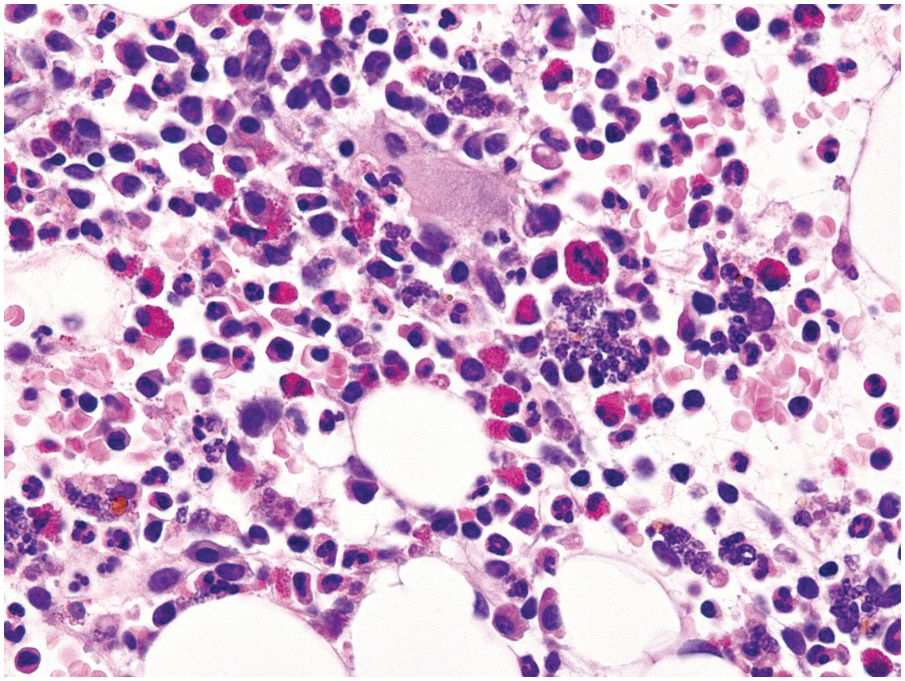
One exception to the rule is BM examination in human immunodeficiency virus (HIV)-infected individuals with haematologic abnormalities, mostly cytopaenias, and in whom virus status is already known [6]. Although easily perceptible, the morphological BM abnormalities in HIV patients are non-specific and range from mild hypercellularity, through overtly dysplastic changes, particularly small pyknotic megakaryocytes (Figure 6.3), nodular lymphoid hyperplasia and plasmacytosis to gelatinous transformation [1, 7, 8], altogether referred to as HIV-associated myelopathy. Some antiretroviral compounds, especially azidothymidine, lead to dysmegakaryopoiesis, megaloblastic erythroid changes and granulocytic dysmaturation [6, 9]. The differential diagnosis from myelodysplastic syndromes based on morphology might be difficult to impossible and requires integration of cytogenetic and molecular genetic data. Special attention must be paid to avoid missing opportunistic infections and lymphomas [10] in the setting of HIV, particularly mycobacterioses (Mycobacterium avium intracellulare) and mycoses (Histoplasma capsulatum, Cryptococcus neoformans) [4, 11]. Granuloma formation may be absent and thus special stains for acid-fast bacilli and fungi (Grocott silver impregnation, mucicarmine and periodic acid–Schiff stain (PAS)) must be considered from the very beginning in BM biopsies of HIV patients (Figure 6.4). Particular attention should be paid to parvovirus B19 inclusions in the erythroid precursors and Epstein–Barr virus (EBV)-infected immunoblastic proliferations in the BM biopsies of HIV+ individuals. Immunohistochemistry for parvovirus B19 and in situ hybridization for EBV-associated small RNA (EBER) may be required. Rarely, human herpesvirus 8 (HHV8)-associated lesions will be detected in the BM of HIV patients, also demonstrable by immunohistochemistry (Figure 6.5) [12]. For more detailed information on BM findings in the setting of HIV-infection reference should be made to Chapter 18.
Figure 6.3 Human immunodeficiency virus-associated myelopathy with atypical pyknotic megakaryocytes, megaloblastic changes of the erythropoiesis, plasma- and lymphocytosis, siderosis and interstitial necrosis. These changes are most likely attributable to both the complex toxic effects of the viral infection and the applied multidrug therapies.
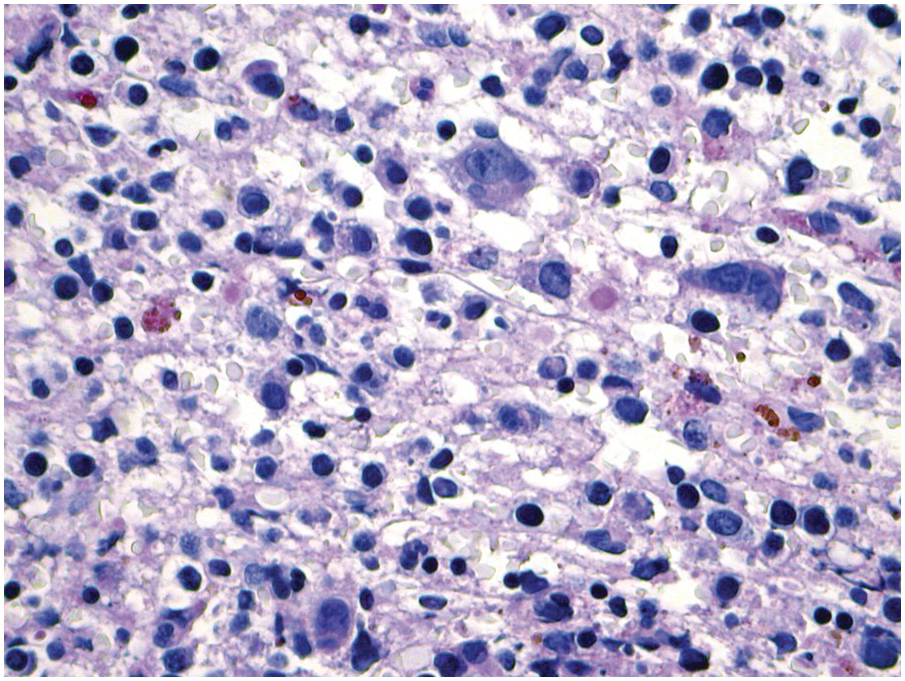
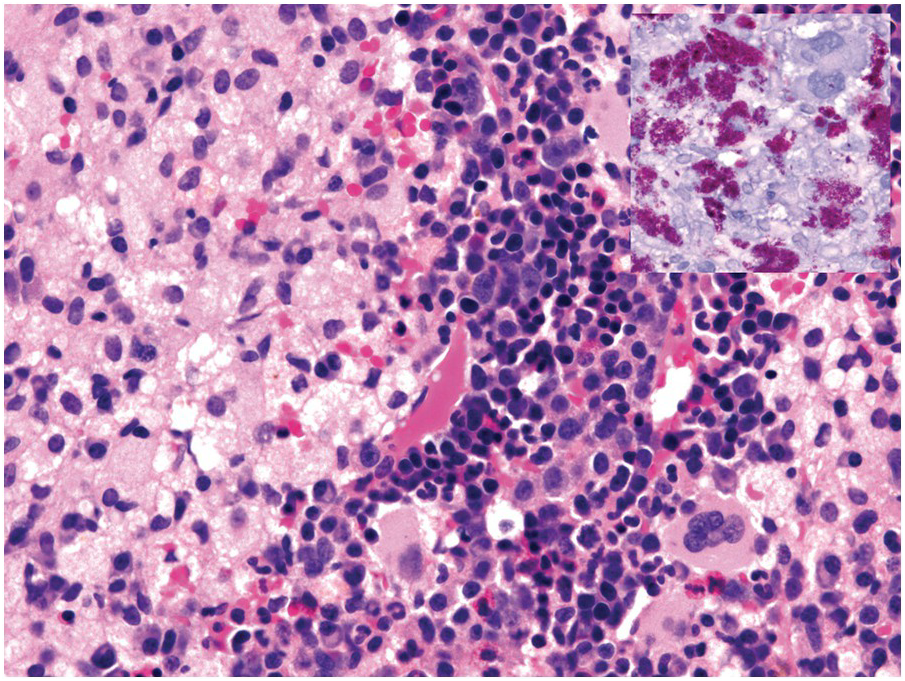
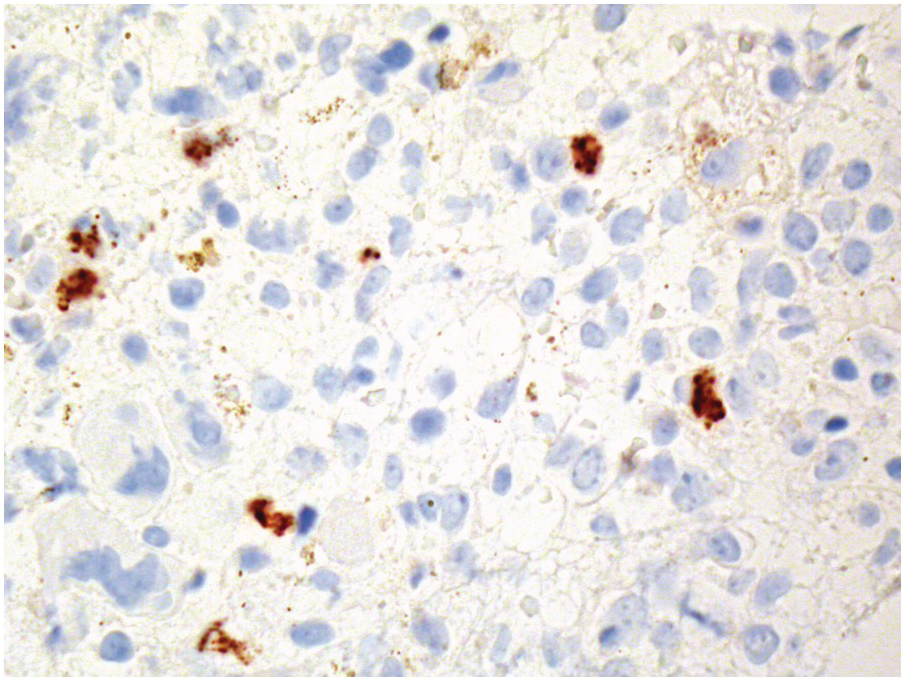
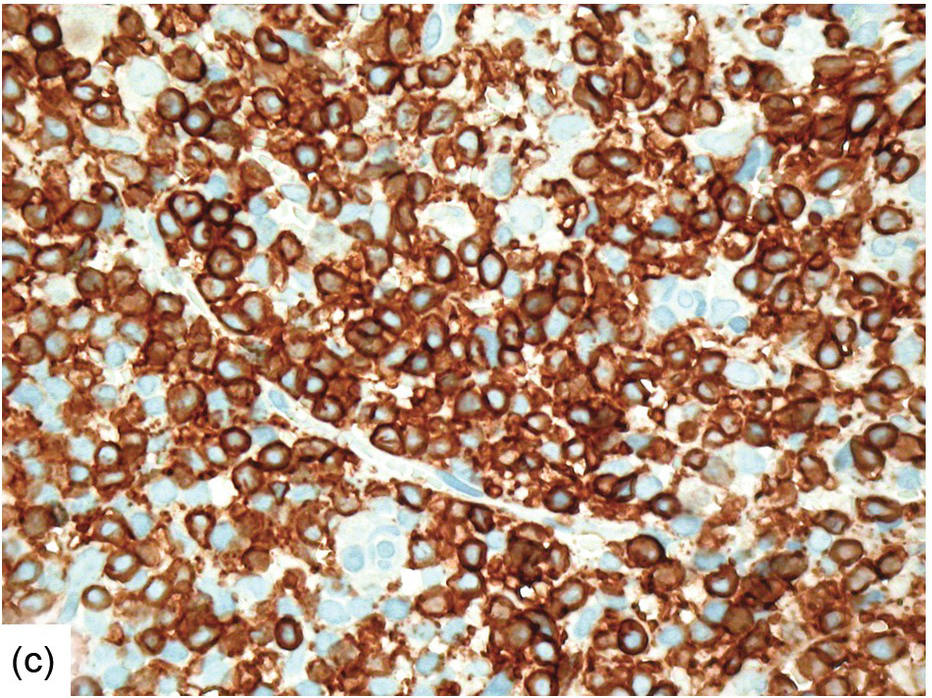
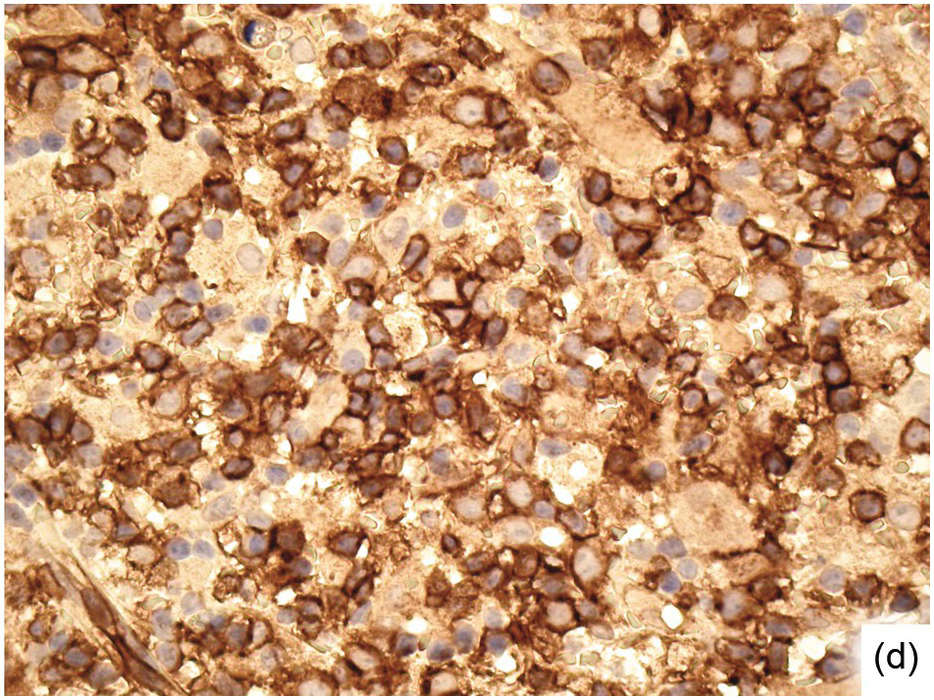
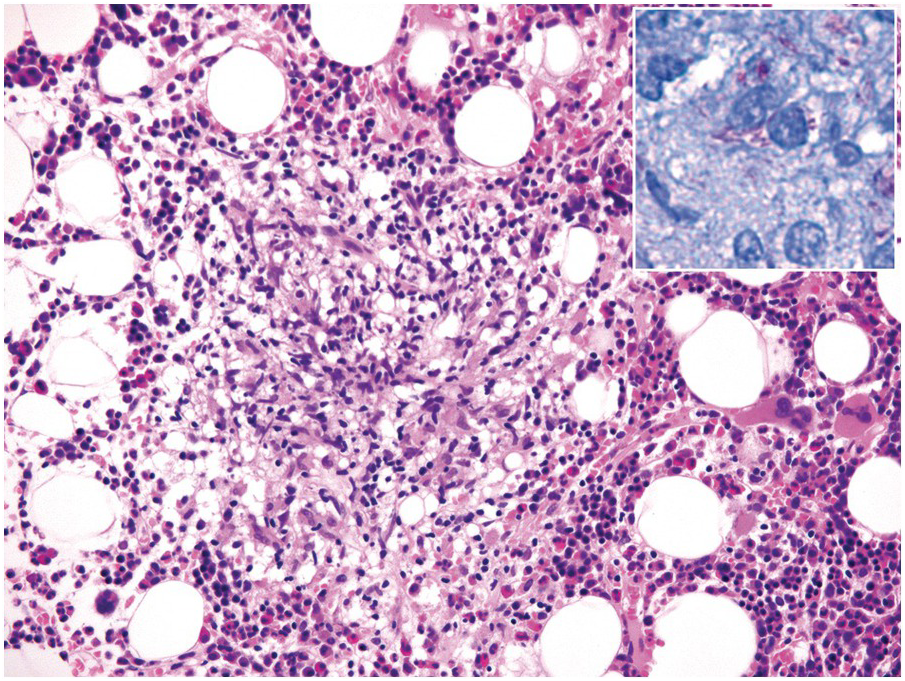
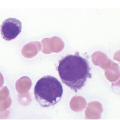
Morphologic BM examinations are rarely performed in acute EBV infection (infectious mononucleosis) in immunocompetent individuals, unless the clinical course is unexpected or the disease is not suspected [13]. The findings are non-specific and include a CD8-skewed, T-cell predominant lymphocytosis, histiocyte increase, plasmacytosis with plasmablasts and, occasionally, vague granuloma formation up to fibrin rings [14]. In situ hybridization for EBER, preferably EBER/CD20 or EBER/CD79a and EBER/CD3 double stains, will detect scattered positive virus-infected B-cells (Figure 6.6a–d). Importantly, detection of EBV in T-cells is almost always pathologic and must raise suspicion of chronic active EBV infection (CAEBV) of the T-cell type, EBV-related haemophagocytic lymphohistiocytosis (see later) or T- or NK-cell lymphomas [15].
(a) Bone marrow in acute infectious mononucleosis with increased interstitial histiocytes, some with visible haemophagocytosis (left-handed figure border), lymphocytes and plasma cells as well as with pronounced myeloid hypoplasia.
(b) Epstein–Barr virus (EBV) infected non-T-cells in the bone marrow of the same patient. Note the presence of EBER– T-cells (brown cytoplasmic staining with nuclear negativity) and EBER+ non-T-cells (i.e. B-cells) with dark blue nuclei without CD3+ cytoplasmic rims.
(c) Significant CD8+ lymphocytosis in the same patient.
(d) CD4 staining for comparison. Note the weakly staining histiocytes.
However, BM examinations for EBV-related diseases in immunosuppressed individuals are often performed. The morphologic changes are as variable as the diseases themselves, ranging from the presence of single, scattered or grouped EBV-infected B- or T-cells in cases of CAEBV (Figure 6.7), through BM depletion with increased histiocytes and variable haemophagocytosis [4], to substantial infiltration by atypical EBV-infected B- and especially NK or T-cells in cases of malignant lymphoproliferations [15]. A diagnosis of Hodgkin lymphoma must also be considered, particularly if EBV-infected single large cells are embedded in nodular, often fibrotic, histiocyte-rich infiltrates (see later).
Figure 6.7 Epstein–Barr virus (EBV) infected T-cells in the bone marrow of a patient suffering from chronic active EBV-infection of the T-cell type. Note the presence of EBER– T-cells (brown cytoplasmic staining with nuclear negativity) and EBER+ T-cells (dark blue nuclei with surrounding brownish cytoplasms).
In cases of cytomegalovirus (CMV) infection BM findings include lymphoid aggregates, myelosuppression and (fibrin ring-) granulomas (Figure 6.8), while typical endothelial viral inclusions are rarely seen [16]; similar nuclear inclusions may be detected in human herpesvirus 6 (HHV6) infection [17]. Immunohistochemistry might be slightly more sensitive than conventional morphologic examination but is rarely indicated considering the much more sensitive blood tests [18]. Importantly, in infants CMV infection may mimic juvenile myelomonocytic leukaemia due to their analogous hypersensitivity to granulocyte-macrophage colony stimulating factor [19].
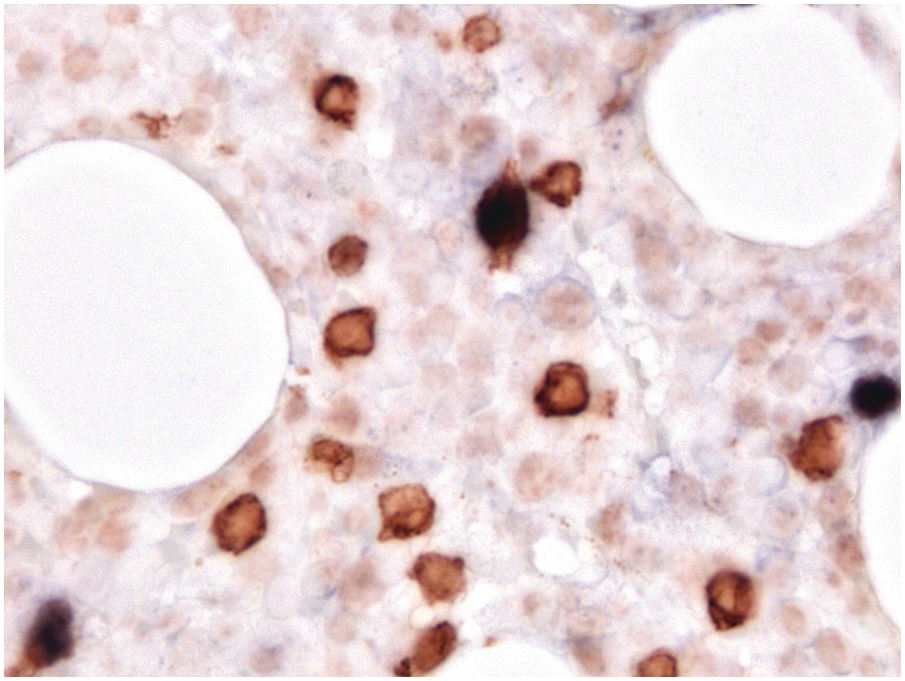
Parvovirus B19 has selective tropism to the erythroid precursors and is directly toxic to the infected cells, causing transient – mostly clinically inapparent – arrest of erythropoiesis [20]. In patients unable to mount adequate antibody titres, i.e. the immunosuppressed, or in patients with preexistent haemolytic disorders such as thalassaemia, sphaerocytosis or other haemolytic anaemias, parvovirus B19 infection can severely exacerbate anaemia. The classic BM findings include erythroid hypoplasia with infected erythroblasts with characteristic nuclear inclusions (Figure 6.2b) that can be verified by application of immunohistochemistry [21], which is in my personal opinion less sensitive than morphology, and by sequencing-based testing. In late-stage disease and in immunosuppressed patients there is an erythroid hyperplasia [22].
Bacterial Infections
Work-up for suspected acute bacterial infection rarely includes BM examination. In specific clinical settings, particularly in immunosuppressed individuals and in patients with long-lasting unexplained fever of unknown origin, possibly with anaemia, BM examinations for a differential diagnosis of chronic infection versus myelodysplastic syndromes are regularly performed.
Mycobacterioses, although rare, are probably the most commonly observed bacterial infections of the BM and comparative studies suggest that BM biopsy examination is superior to other techniques in this diagnosis [23]. Mycobacteria usually provoke a granulomatous response, which varies from well-formed granulomas with little caseous necrosis to loose collections of histiocytes with huge areas of necrosis [4]; the latter is particularly prominent in severely immunocompromised patients (the lower the CD4+ counts or the membrane-bound tumour necrosis factor α (TNFα), and the more virulent the mycobacteria – the looser the granulomas). In immunosuppressed individuals Mycobacterium avium intracellulare is the most common species [24], while in patients who experienced infection due to contaminated heater–cooler units used in cardiac surgery Mycobacterium chimaera [25, 26] can be detected (Figures 6.4 and 6.9). Irrespective of the species, application of acid-fast stains (Ziehl–Neelsen, Wade–Fite), supported by sequencing-based techniques is mandatory to diagnose and subtype BM mycobacterioses, which includes a range of rare types.
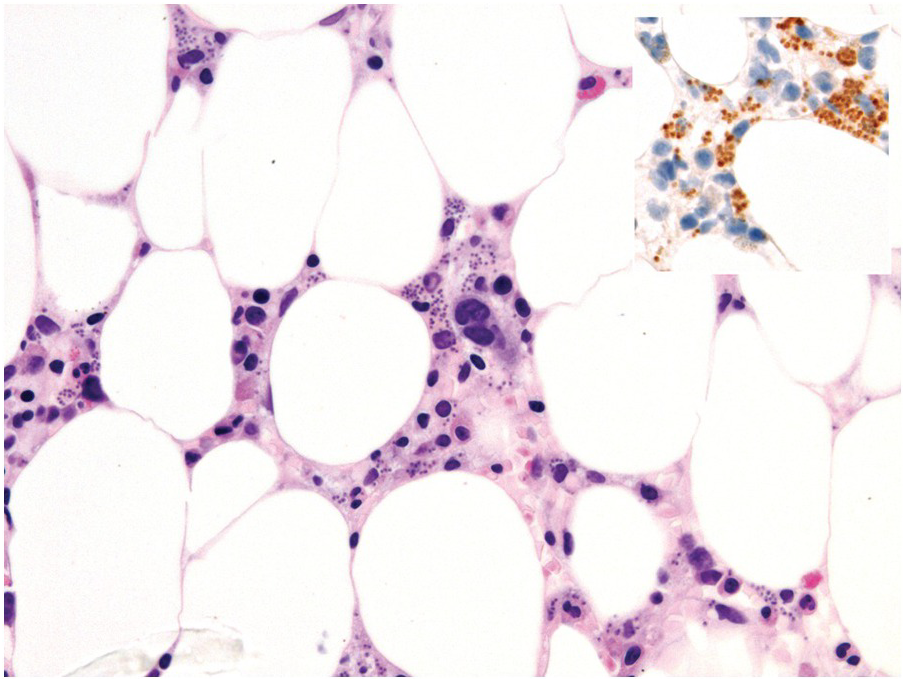
The BM can be a site of involvement by Whipple disease [27]. Typically non-caseating, vague epithelioid granulomas with foamy macrophages or scattered single foamy cells containing diastase-resistant PAS+ organisms are seen (Figure 6.10). In any case of suspected Whipple disease sequencing-based testing of the respective tissue should be performed [28].
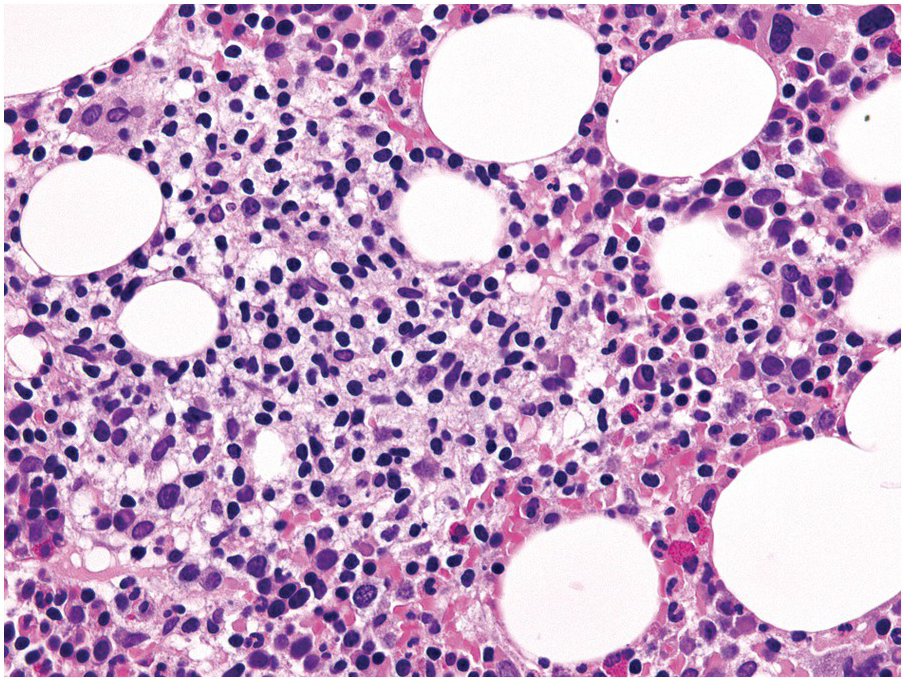
Rickettsioses, Q-fever (caused by Coxiella burnetii), salmonelloses, borreliosis and brucellosis can be accompanied by a considerable BM lymphocytosis and (fibrin ring-) granulomas (Figure 6.11). While the respective agents are hardly, if ever, morphologically detectable, application of a specific ancillary test can help in establishing the exact diagnosis. Cat-scratch disease involving the bone/BM, admittedly more probably being a kind of osteomyelitis, can be accompanied by (fibrin ring-) granulomas as well as by the typical suppurative-granulomatous changes analogous to those seen in affected lymph nodes, and organisms may be detectable by application of the Warthin–Starry silver impregnation technique [29, 30].
Fungal Infections
The incidence of systemic mycoses is increasing due to the escalating number of patients who are immunocompromised because of diabetes mellitus, haemodialysis, organ and haematopoietic stem cell transplantation, chemotherapy for cancer and infection with HIV, as well as due to the broad use of antibiotics and immunosuppressive drugs [31]. The diagnosis of fungal infections is based on histology, culture, and radiologic and serologic tests with various sensitivities and specificities [32, 33]. Histological proof of mycosis is regarded as very reliable, since the pathogenic agent can be unequivocally detected in the affected tissues [1]. BM biopsy analysis might be a useful tool for examination of cryptic infections, especially in HIV-patients, with an overall diagnostic yield of 32% and 6% for infectious and fungal diseases, respectively [34]. So far, isolated BM mycoses in non-neutropaenic, immunocompetent patients, without evidence of fungaemia or septicaemia, have only occasionally been reported [35, 36].
Typically, fungal infections in the BM display histiocytic hyperplasia with granuloma formation and necrosis [1]. The most common BM mycoses include histoplasmosis and cryptococcosis [1, 4, 11]. To identify fungi, special attention should be paid to the PAS and Gömöri stains, which are usually performed on BM biopsies as part of the routine diagnostic work-up, since the organisms are readily visible if careful examination is undertaken (Figures 6.2a and 6.12). In addition, special stains such as Grocott silver impregnation and a mucicarmine stain should be considered. Particular attention must be paid to distinguish Histoplasma from neutrophils ingested by macrophages – especially in circumstances with high neutrophilic counts such as chronic septic conditions and chronic myelomonocytic, chronic myeloid and chronic neutrophilic leukaemia – that can mimic fungi (Figure 6.13).
Figure 6.13 Decaying phagocyted granulocytes in the bone marrow of a patient suffering from pneumonia. Note nuclear debris that may be mistaken for Histoplasma.
Other Parasitic Infections
The most common protozoal infections of the BM are leishmaniasis and toxoplasmosis. Toxoplasmosis is more commonly observed in immunocompromised individuals and both are characterized by hypercellularity, variable fibrosis and granuloma formation, necrosis and visible parasites (amastigotes), either in macrophages/monocytes or, rarely, disseminated in the interstitium (Figure 6.14) [37, 38]. Organisms may be scarce. Particular attention must be paid to distinguish parasites from platelets, which can be achieved by high-power microscopy to identify characteristic intracellular organelles (nucleus, kinetoplast etc.) within parasites, as well as by applying the MTB1 anti-CD1a antibody-clone, which shows cross-reactivity with Leishmania spp. [39], and the anti-toxoplasma-antibody.
Benign Histiocytic Disorders of the Bone Marrow
Depending on whether histiocytes form secondary structures called granulomas or not, reactive histiocytic diseases of the BM (and elsewhere) [39] can be separated into diffuse and granulomatous histiocytic disorders, which has major practical implications as exemplified later. Important diagnostic and differential diagnostic considerations of BM patterns linked to benign histiocytic disorders are summarized in Table 6.2.
Table 6.2 Histopathologic pattern-based diagnostic and differential diagnostic considerations in granulomatous and benign histiocytic bone marrow disorders.
| Bone marrow patterns with histiocytoses | Probable/possible diagnoses | Differential diagnoses | Ancillary techniques |
|---|---|---|---|
| Lipogranulomatous changes | Non-specific, common in infections | Erdheim–Chester disease, Whipple disease | BRAF (VE1)-stain, sequencing Diastase-resistant PAS stain |
| Epithelioid granulomas – caseous granulomas – fibrin ring granulomas – foreign body granulomas – sarcoid-type granulomas – perivascular | Infections, chronic granulomatosis* Infections (see text body) e.g. Displaced keratin or chondroid Drug reactions, infections, immunologic disorders*, sarcoidosis Granulomatous polyangiitis, syphilis | Septic changes, tumour necrosis, Paget’s disease of bone Lymphomas masked by or provoking granulomas Lymphomatoid granulomatosis | Histo- and immunohistochemistry Serology & Sequencing Imaging studies *Constitutional genetic analyses (according to and designed to yield the suspect diagnosis) |
| Histiocyte increase – bluish (on Giemsa- stained slides) – crystal-storing – diastase-resistant PAS+ – diffuse, cytologically unremarkable – foamy/vacuolated – granular – haemophagocyting – iron+ – sea blue – with emperipolesis – with foreign substances | Gaucher disease*, increased turnover Crystal-storing histiocytosis Whipple disease Infections (see text body), immunologic disorders* After myeloablative therapy, dyslipidemias*, lysosomal storage diseases* Mycobacterioses, parasitoses Haemophagocytic lymphohistiocytosis* Iron overload conditions* Sea-blue histiocytosis* Rosai–Dorfman disease (of bone) Crystal-deposition disorders* | BCG-vaccination, infections, myeloproliferative neoplasms Myeloid (histiocytic) neoplasms Erdheim–Chester disease, fat necrosis, polyvinylpyrrolidone application Rosai–Dorfman disease (of bone) Non-specific haemophagocytosis Formalin (pigment) artefacts Myeloproliferative neoplasms Non-specific, septic conditions | Histo- and immunohistochemistry e.g. BRAF (VE1)-, Giemsa-, Grocott-, PAS/diastase-resistant PAS-, Wade–Fite-, Warthin–Starry- and Ziehl–Neelsen stains Serology & Sequencing *Constitutional genetic analyses (according to and designed to yield the suspect diagnosis) |
Granulomas in the Bone Marrow
Microscopic aggregations of epithelioid histiocytes are referred to as granulomas and the respective inflammatory process is called granulomatous [40–42]. Granulomas show variable morphologic features including central areas of necrosis (necrotizing granulomas) or suppuration (microabsceding or suppurative granulomas), incorporation of foreign material or presence of giant cells due to the fusion of macrophages. Granuloma formation is usually associated with local CD4+ T-cell activation (and numeric increase of CD4+ T-cells at the site of granuloma formation, thus ‘consuming’ CD4+ T-cells and occasionally leading to skewing of the CD4/CD8 ratio in the peripheral blood in favour of CD8), and production of interleukin 2 and 12, interferon γ (IFNγ) and TNFα [43]. In general, granulomas are provoked by agents that are difficult to eradicate by the enzymes of histiocytes, either due to the different phospholipid composition of the former (e.g. mycolic acids) or due to the specific enzymo-/immunogenetic background of the host.
In contrast to other inflammatory morphologic patterns, the number of causative agents in granulomatous responses is rather small and granulomas are therefore considered to be ‘specific’. Narrowing down the possible causatives, the identification of granulomas and the subsequent search for possible underlying agents and conditions finally supports establishing more precise clinicopathological diagnoses.
Morphology of Bone Marrow Granulomas and Aetiologic Considerations
Granulomatous processes involving the BM can be divided into three subgroups based on morphology and clinicopathological context: (1) lipogranulomas, (2) infectious epithelioid granulomas and (3) epithelioid granulomas associated with immune dysregulation. Importantly, most inborne immunodeficiency disorders such as Blau syndrome, common variable immunodeficiency, recombination-activating genes, X-linked inhibitor of apoptosis protein-deficiency and chronic granulomatous disease are accompanied by increased granuloma formation [44]. Depending on whether solely the BM or other organs are also involved, granulomatous BM processes may represent isolated findings or reflect involvement of a systemic disorder. It is obvious that detection of a granulomatous BM process should be followed by an integrative diagnostic work-up considering the clinical history (evidence of inborne immunodeficiency, travelling and drug history) and presentation, imaging and molecular detection methods, including serology, in situ- and sequencing-based procedures.
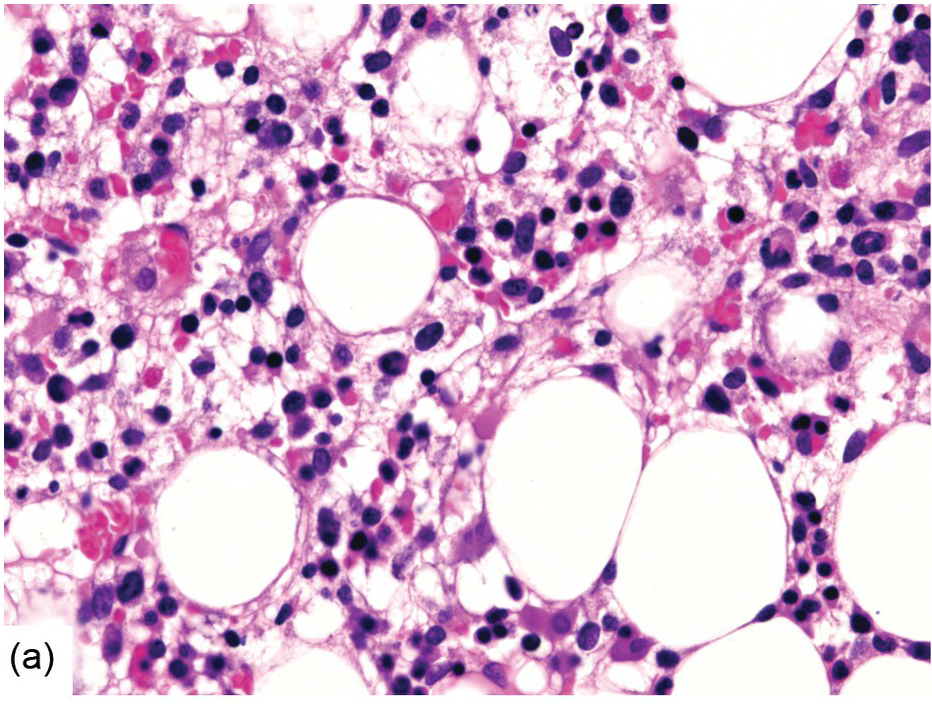
Lipogranulomas
Lipogranulomas are found in up to 10% of BM samples. They are not thought to be significant since they are probably not linked to specific underlying disorders. However, they may be more commonly observed in patients with acute febrile illnesses. They consist of aggregates of histiocytes with variably sized lipid vacuoles (Figure 6.15), which tend to gradually disappear with time resulting in a morphology that is indistinguishable from epithelioid granulomas. When investigating BM lipogranulomas, special attention must be given to the PAS/diastase-PAS stains so as not to miss involvement by Whipple disease, in which the foamy histiocytes stain positively (Figure 6.10); any suspected case of Whipple disease should be investigated by sequencing-based procedures. Granulomas in Erdheim–Chester disease (see later) may sometimes resemble lipogranulomas [45].