Abstract
The bone marrow (BM) is a frequent site of haematogenous spread for all types of cancer. Metastatic spread of disseminated tumour cells (DTCs) to the BM is detected in 0.2 to 12% of patients with solid tumours [1]. The variability in incidence is related to the incidence of the primary tumour and its homing behaviour [2]. Common primary tumours affecting the BM are listed below (Table 17.1).
Definition
The bone marrow (BM) is a frequent site of haematogenous spread for all types of cancer. Metastatic spread of disseminated tumour cells (DTCs) to the BM is detected in 0.2 to 12% of patients with solid tumours [1]. The variability in incidence is related to the incidence of the primary tumour and its homing behaviour [2]. Common primary tumours affecting the BM are listed below (Table 17.1).
Table 17.1 Common primary tumours metastatizing to the bone marrow [2].
| Paediatric | Adult (Figures 17.1–17.5) |
|---|---|
| Neuroblastoma Retinoblastoma Ewing sarcoma Rhabdomyosarcoma Medulloblastoma | Breast cancer Prostate Lung Gastrointestinal carcinoma |
Involvement of the BM by metastatic tumour adversely affects the response to treatment and overall survival. The results of a retrospective study showed that the median survival time post symptomatic BM involvement in breast cancer patients was 2.6 months. Moreover, it is an indication of metastasis to other sites [3]. The role of FDG/PET-CT in detection of BM involvement is increasingly recognized. However, BM examination is still a cornerstone in the diagnosis of metastatic lesions, with increasing interest in employing modern diagnostic techniques for BM micrometastases.
Pathogenesis
The metastatic cascade of events starts with tumour proliferation and growth, followed by tumour migration and spread and, finally, clinical presentation or clinical dormancy. Accumulating data has provided increasing evidence that metastatic spread often occurs before the detection of the primary tumour [4]. The BM is one of the common organs for homing of the disseminated tumour cells (DTCs). The DTCs could remain dormant for years and present with a late recurrence [5].
There is an intermediate step between tumour proliferation and dormancy; the step of dissemination of circulating tumour cells (CTCs) via the blood-stream or lymphatic stream. The CTC spreads passively or actively until it finds its premalignant niche. Then a CTC becomes a DTC and clinical cancer dormancy begins [5].
The mechanism of formation of a malignant niche in the BM is still not clearly understood. The main hypothesis behind metastatic involvement of BM starts with the attraction of the DTCs to the BM (i.e. homing), followed by interactions between the DTCs and the BM microenvironment (forming a malignant niche), and finally proliferation and survival of the tumour cells in the BM [6, 7].
This hypothesis explains the pathological findings and clinical outcome of metastatic BM involvement. The homing molecules expressed on the DTCs and the BM microenvironment differ according to the primary tumour. For example, the SDF1/CXCR4 is thought to be responsible for homing of prostatic and breast carcinoma, neuroblastoma and rhabdomyosarcoma. The settling of the DTCs in the BM is mediated by the interaction of the former with the mesenchymal and vascular niches. This promotes tumour proliferation and cytokine release, which results in a florid stromal reaction and bone remodelling [6, 7].
Formation of the malignant niche promotes evasion of DTCs and resistance to chemotherapy and radiotherapy. Eventually the malignant niche overrides the BM microenvironment resulting in haematological abnormalities [6].
Clinical Syndromes
Clinical presentations of metastatic bony infiltration are very diverse and are influenced by the primary tumour, performance status of the patient and the stage of the disease. Common clinical presentations are outlined in Table 17.2.
Table 17.2 The different clinical presentations of bone marrow metastasis [8].
| Related to the primary tumour | Related to infiltration of the bone | Abnormal radiographic findings | Haematological |
|---|---|---|---|
| Specific symptoms related to the primary tumour | Bony pains | Abnormal hot spots on isotopic bone scans or PET scanning | Symptoms and signs of anaemia |
| Cachexia | Pathological fractures | Abnormal MRI | Bleeding related to thrombocytopaenia |
| Hypercalcaemia | |||
| Elevated serum alkaline phosphatase activity |
The Peripheral Blood Smear
Haematological findings can be an early or a late sign of BM metastasis. Some patients can have a normal peripheral blood smear. In others, abnormalities in the peripheral blood smear could be secondary to the primary tumour or to impairment of normal haematopoiesis [8].
The most common abnormality is a normocytic normochromic anaemia, less commonly thrombocytopaenia and neutropaenia [9]. Furthermore, in less than half the patients a leukoerythroblastic blood film is reported, the severity of which is proportional to the degree of marrow fibrosis [8, 9].
The presence of metastatic malignant cells in the peripheral blood is a rare finding. However, carcinocythaemia can be found in small cell carcinoma of the lung, when it is often a terminal event, carcinoma of the breast and malignant melanoma [10, 11]. Circulating tumour cells can rarely be found in small cell tumours in children [8, 11]. Circulating Reed–Sternberg cells and mononuclear Hodgkin cells, occasionally sufficient in number to constitute leukaemia, have also been described [11].
Bone Marrow Examination in Adults
In general, a bone marrow biopsy (BMB) is more conclusive than a clot section and an aspirate smear; as the former shows the spatial arrangement of tumour cells, the degree of involvement and an impression of the haematopoietic reserve. Moreover, aspiration is frequently unsuccessful (dry tap) due to fibrosis. Nonetheless, the examination of all samples is complementary [9].
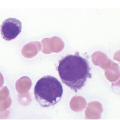
Distinction of non-haematopoietic cells in the BM requires meticulous examination of the BM smear, especially at the tail and feathery edge. They are highly pleomorphic in terms of size and are distinctly larger than all haematopoietic cells apart from megakaryocytes. An exception would be small cell carcinoma of the lung, which is smaller than other carcinomas. Metastatic tumour cells occur in aggregates, sheets or nests, usually coalesced together with moderate to abundant blue cytoplasm and indistinct cellular borders.
Occasionally they occur in singular forms such as in small cell lung carcinoma and in metastatic mammary lobular carcinoma [6]. Therefore differential diagnosis would include blast cells of acute leukaemia, lymphoid malignancy, neoplastic cells of Langerhans cell histiocytosis or systemic mastocytosis. In such cases flow cytometry using CD45 is helpful. The tumour cells can also be multinucleated with evident nuclear moulding and clumping [2, 8].
Cytological distinction and identification of the tumour primary origin based on the aspirate smear is rather difficult. However, malignant melanoma cells can be identified by their strong cytoplasmic melanin pigment. Additionally the presence of tumour cells with abundant clear cytoplasm is distinctive of renal cell carcinoma [2].
Necrosis with ghost cells is frequently seen in metastatic BM aspirates with few or no tumour cells. Gelatinous transformation is extremely rare but is reported in cachectic patients [8]. In a retrospective study three factors were predictive of a metastatic infiltration of the BM: a hypoplastic marrow, the presence of thrombocytopaenia and high serum alkaline phosphatase [1].
In cases of a negative smear and a suspicious clinical context, the presence of eosinophilia, plasmacytosis, increased number of macrophages and increased osteoblasts and osteoclasts, are highly suggestive of underlying BM pathology [2, 9].
Bone Marrow Histological Findings
Histological patterns of the metastatic BM involvement are highly variable. Any combination of stromal or bony reactions, as well as variable cytological features, can be seen (Table 17.3). However, the diagnostic approach to the BMB is essentially the same; outlined in Table 17.4.
| Pattern of infiltration | Focal Diffuse (complete effacement) Mixed |
| Stromal reaction | Fibroblast proliferation and reticulin deposition Inflammatory cellular reaction (increased lymphoid, mast cells and macrophages) Angiogenesis Necrosis |
| Bony changes | Osteolysis Osteosclerosis Mixed |
| Cytological features | Adenocarcinoma (formation of glands, presence of signet ring cells or presence of mucin) Squamous cell carcinoma (formation of keratin or the presence of intercellular bridges) Mixed Singular forms |
Table 17.4 Suggested bone marrow diagnostic approach for adults.
Clinical/laboratory suspicion of bone marrow infiltration
|
An adequate BMB would be 1.5 to 2 cm long with an average diameter of 0.3 cm. Cartilage and periosteum are excluded from length of the sample [6]. In several studies bilateral BMB were superior to unilateral in detecting metastasis. However, this can be troublesome to patients. Thus staged sectioning at defined levels is highly recommended to overcome entrapment of carcinoma cells in fibrosis and the focal behaviour of the infiltrate [2, 6, 9]. Moreover a radiologically assisted/targeted BMB can be performed.
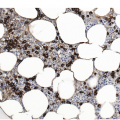
Metastatic infiltration of the BM is accompanied by stromal and bone reactions. Carcinomas (Figures 17.1–17.5) most frequently metastasize to the BM followed by malignant melanomas and, rarely, sarcomas (Figure 17.6) [8].
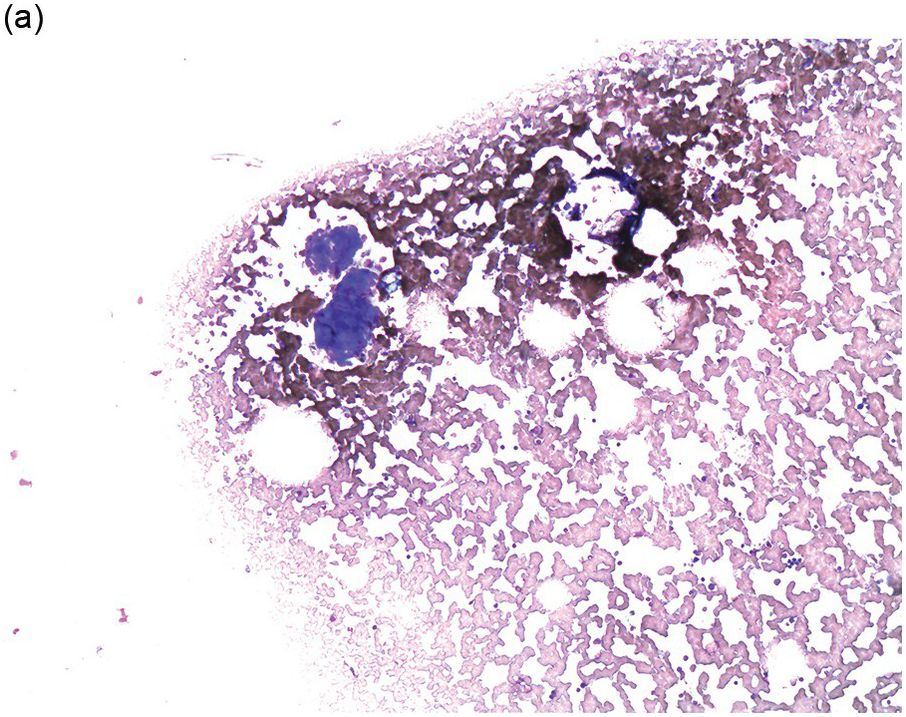
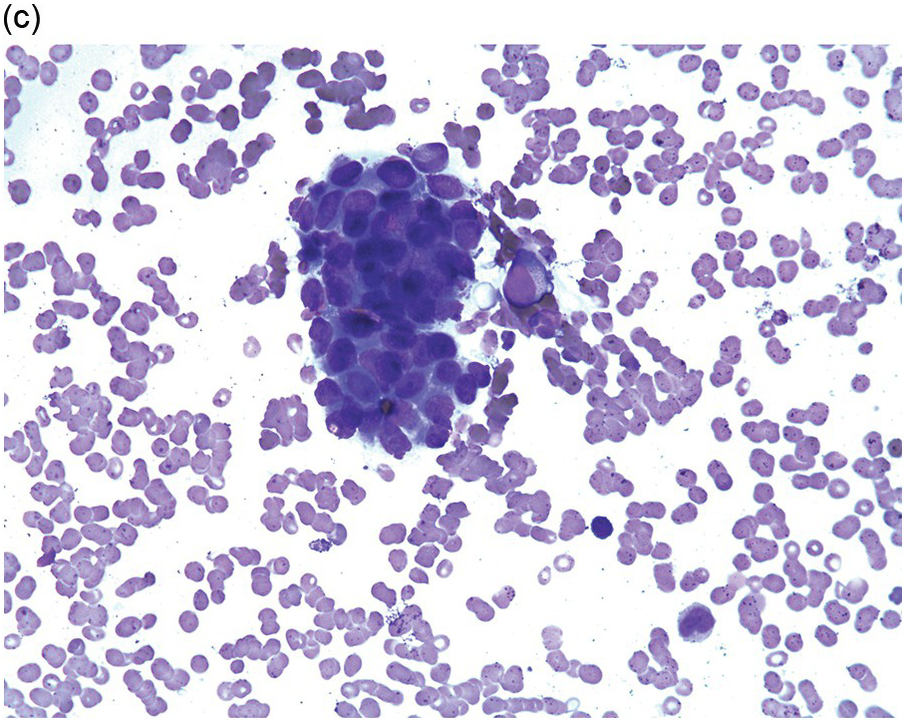
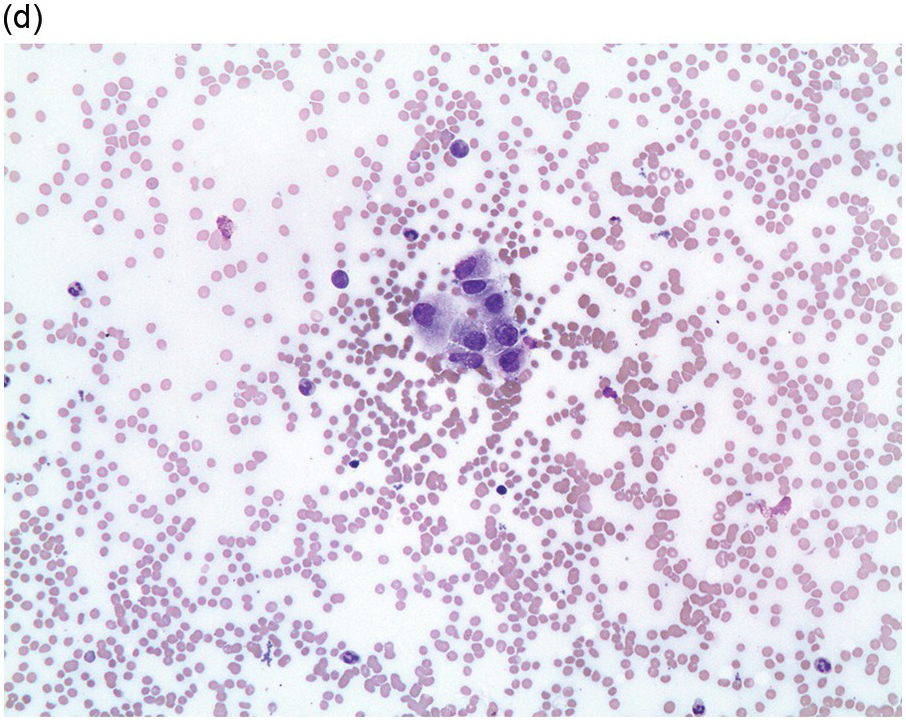
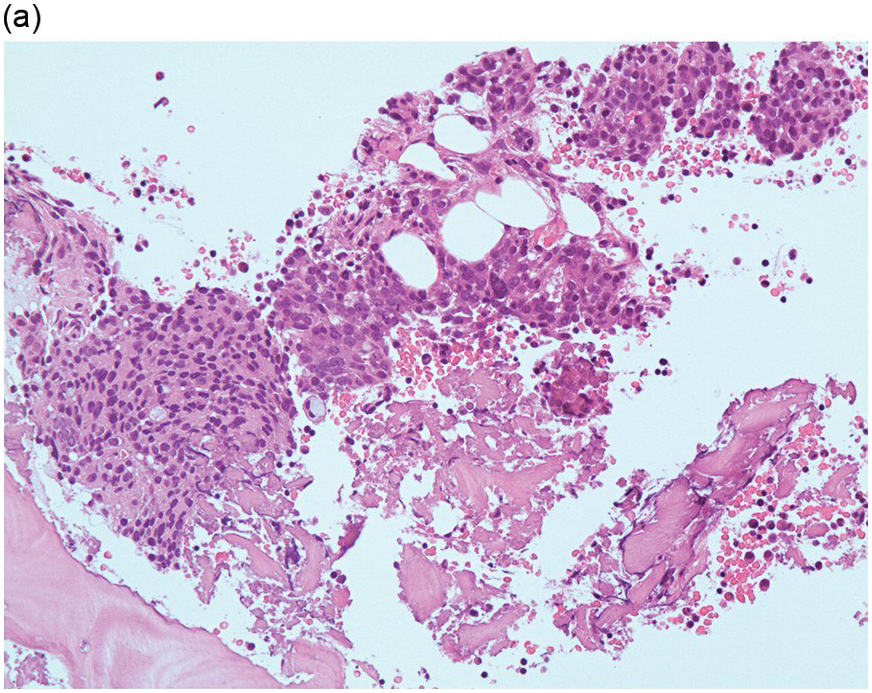
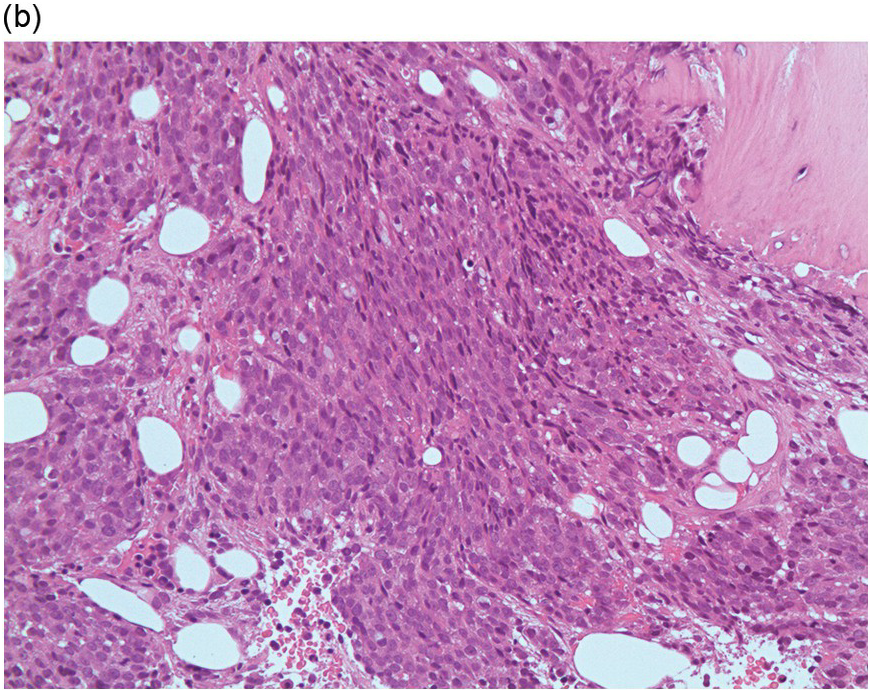
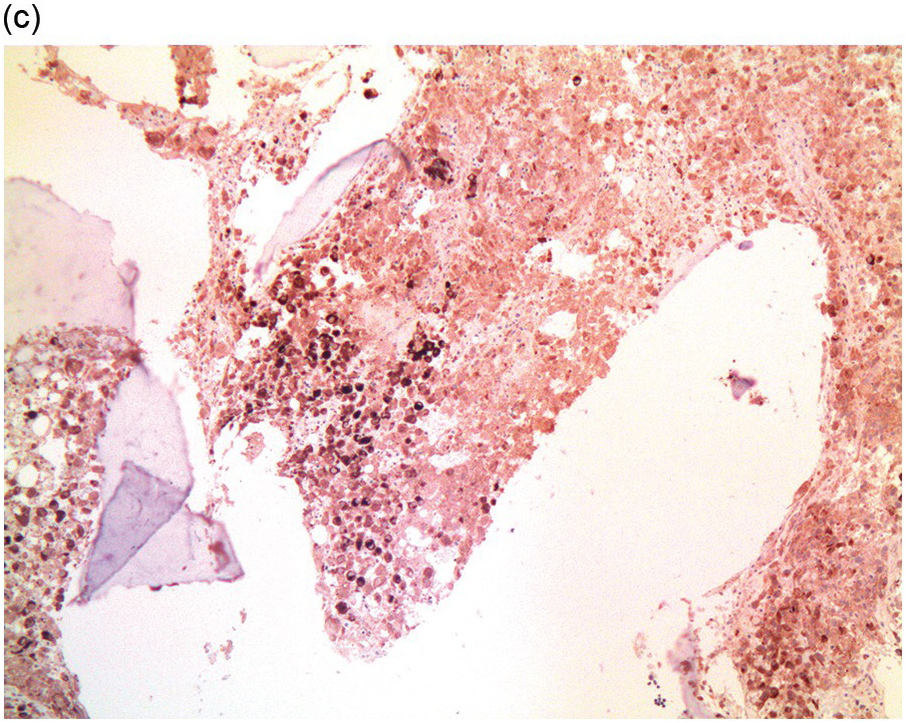
Figure 17.1 (a–c) Metastatic breast carcinoma, ductal NST. Bone marrow aspirate showing cohesive aggregates of large atypical non-haematopoietic cells. (d) Metastasis from lobular breast carcinoma. The cells are large in size and could be seen singly or forming files. (Magnification: a ×10, b × 20, c ×40, d ×20)
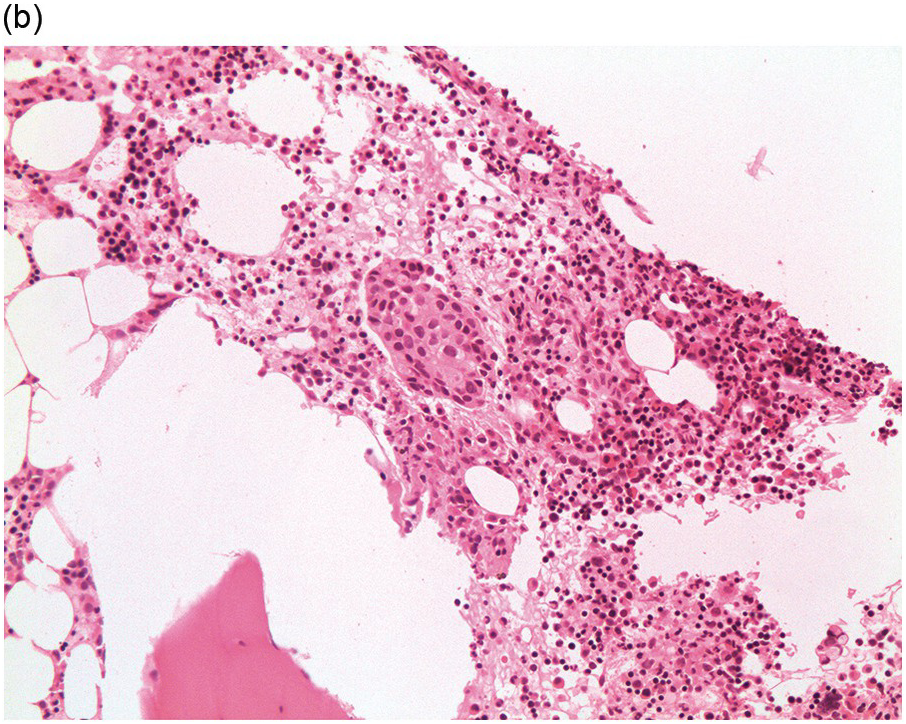
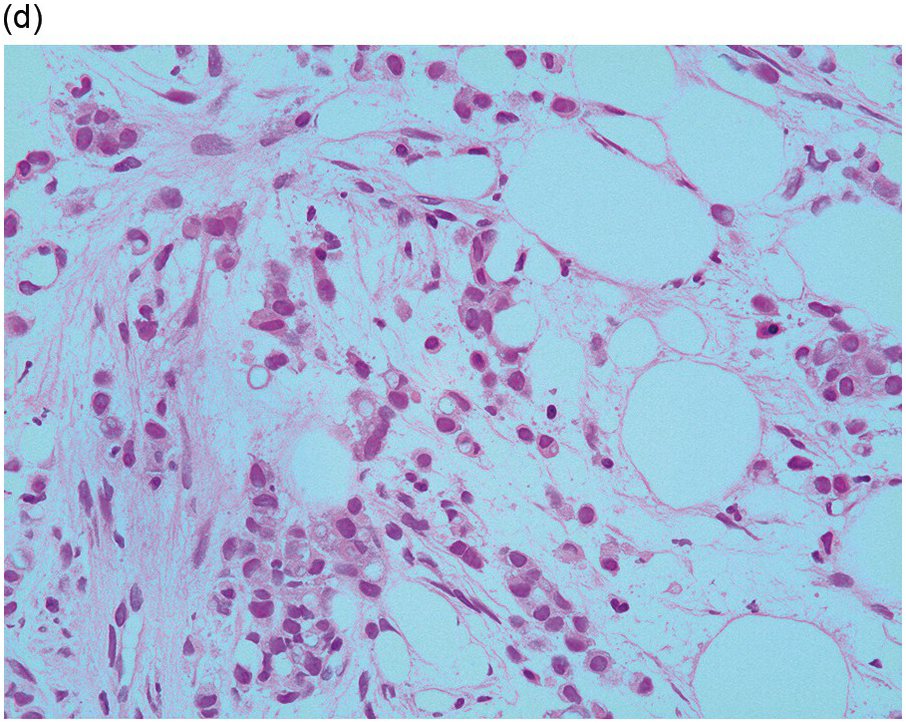
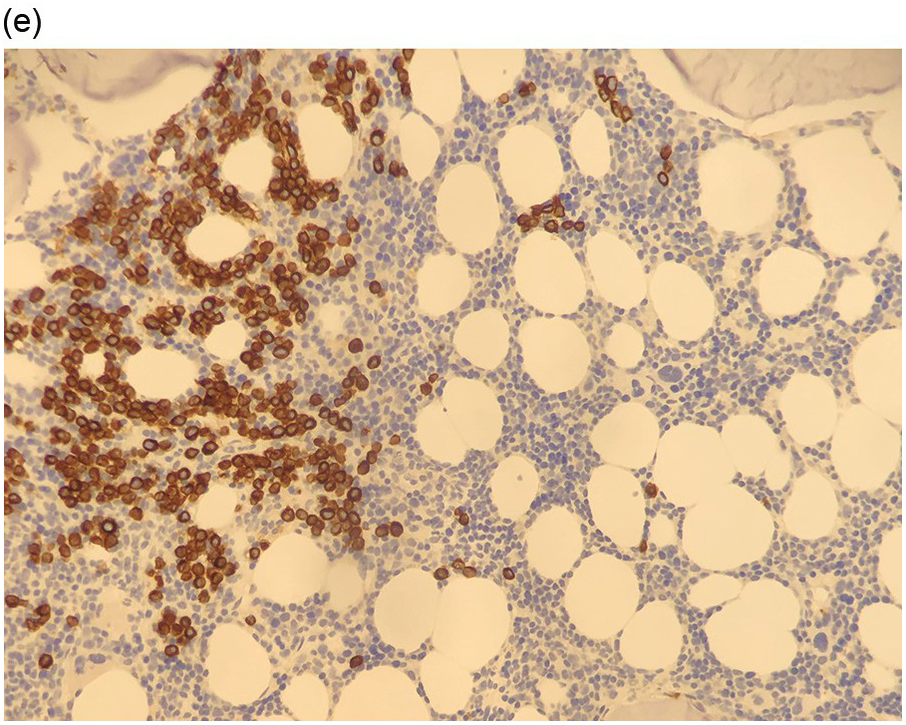
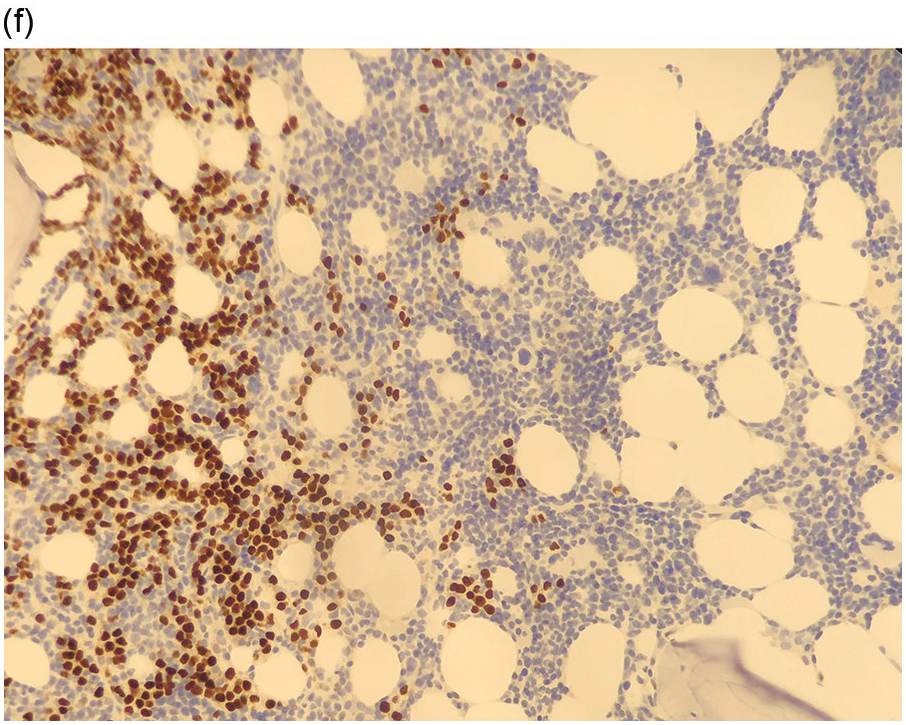
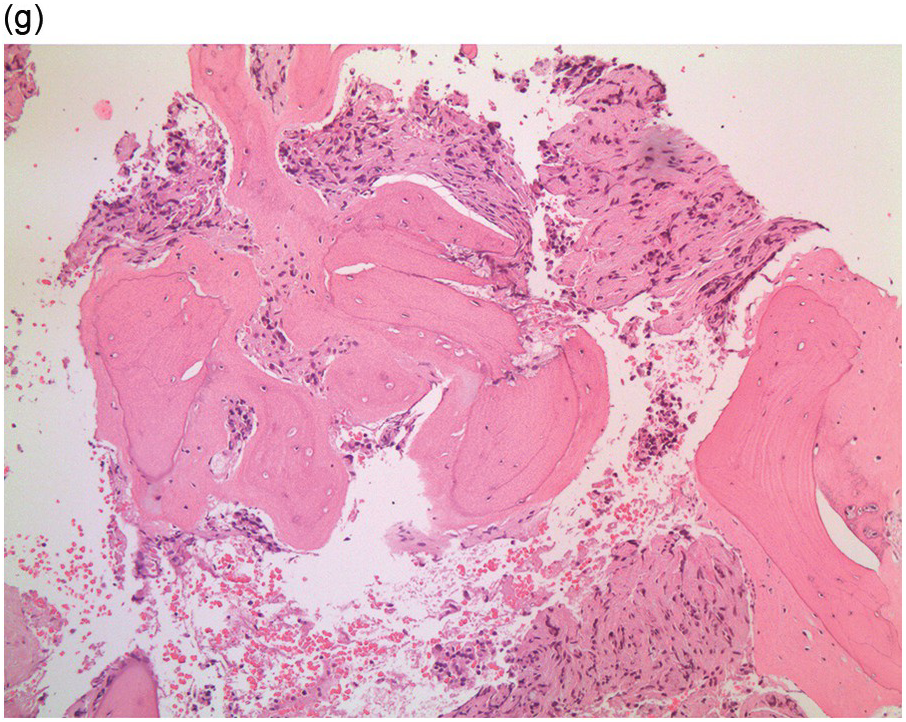
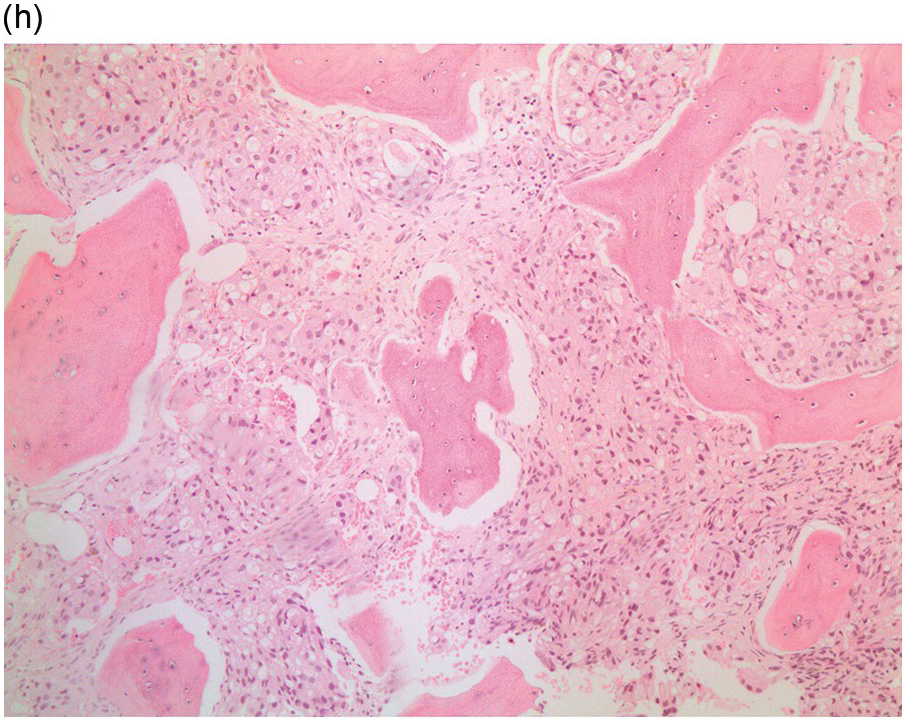
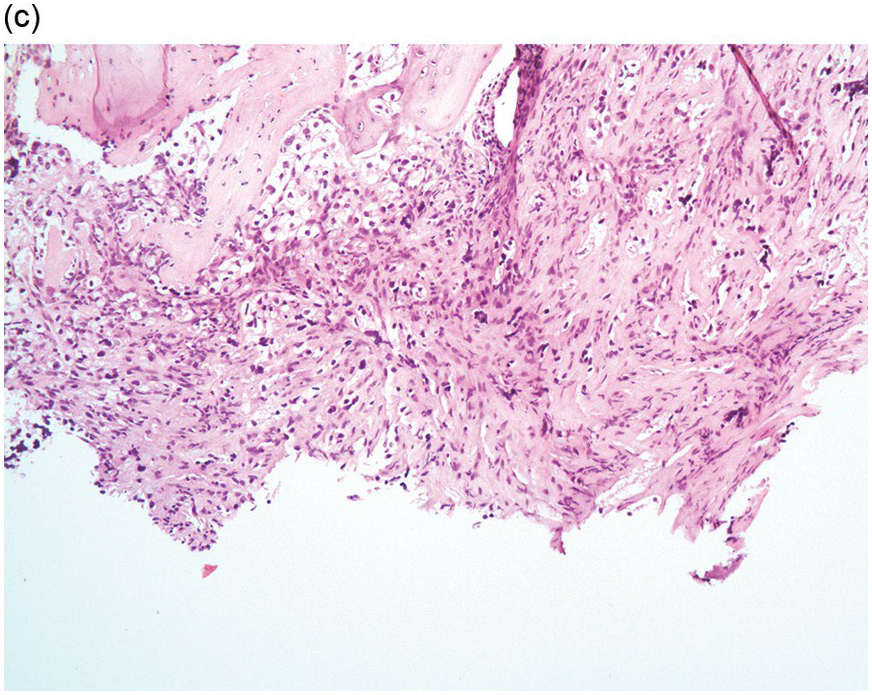
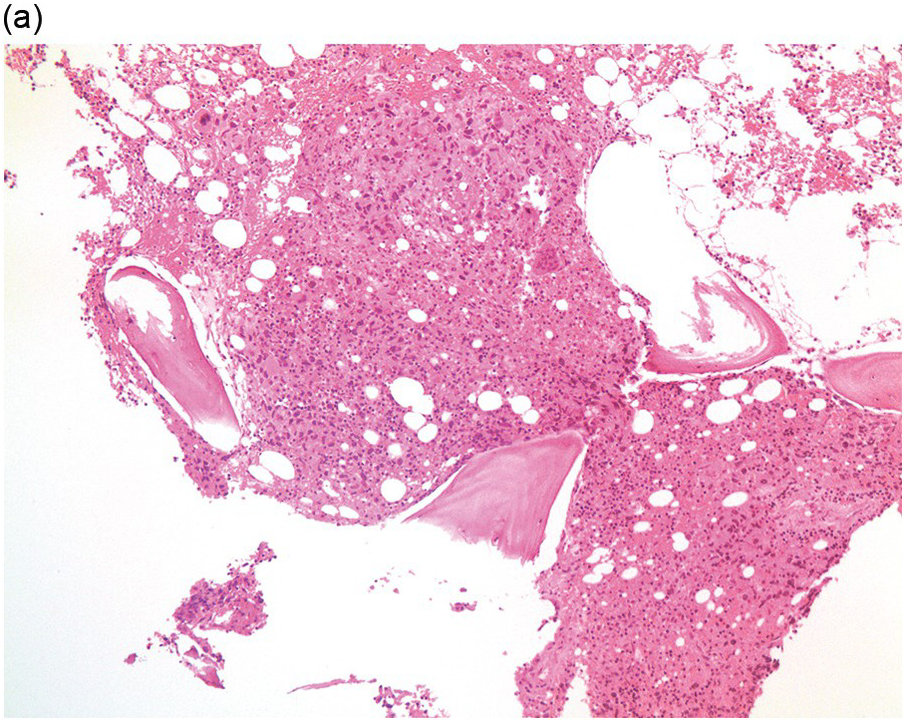
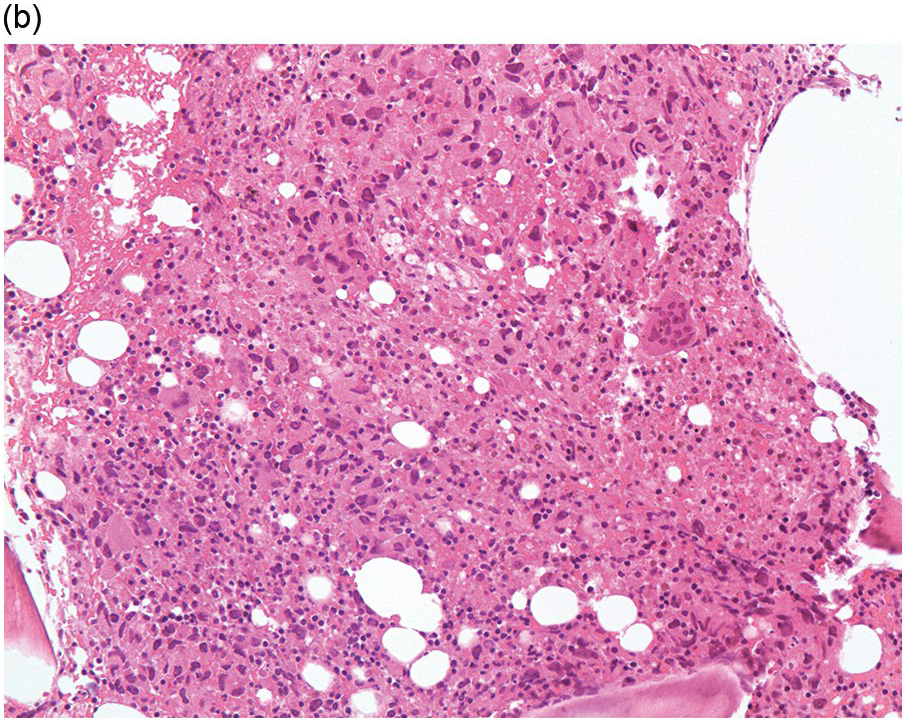
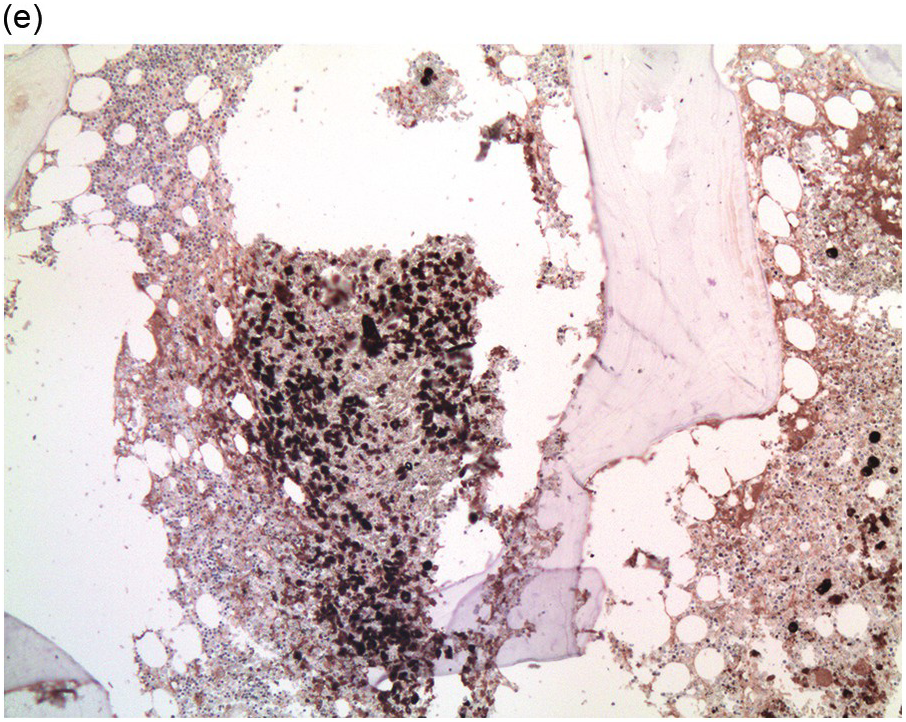
Figure 17.2 (a, b) Sections from bone marrow trephine biopsy showing metastatic breast carcinoma, ductal NST. The tumour cells are cohesive and are forming clusters and glandular structure. (c, d) Metastatic lobular breast carcinoma. (c) The tumour cells are effacing the bone marrow space and are mostly forming Indian files, single cells are also noted. (d) The tumour cells show interstitial distribution and are seen mostly singly with some having signet cell morphology. (e, f) The tumour cells are positive for CK7 (e) and oestrogen receptor (f) and negative for CD45, CK20, CD×2 and TTF1 (not shown). (g, h) Sections of bone marrow trephine biopsy highlighting stromal fibrosis and osteosclerosis. (Magnification: a–d ×20, e–f ×10)
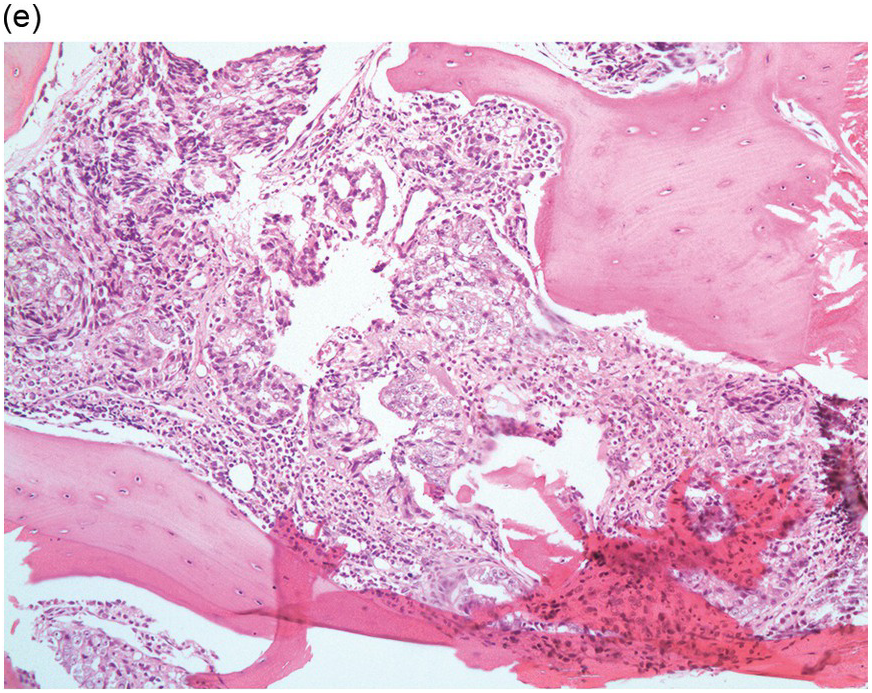
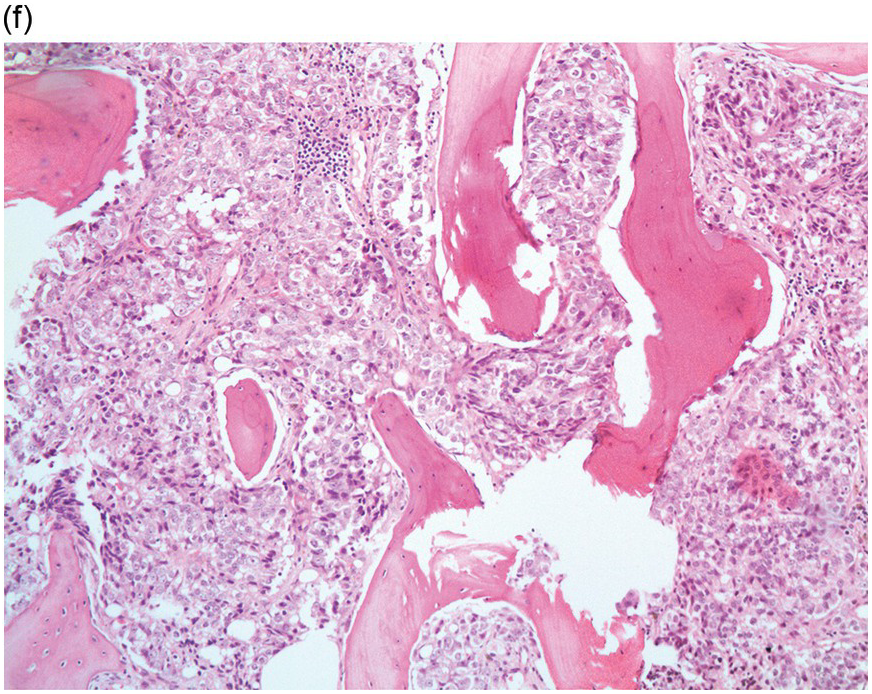
Figure 17.3 (a, b) Bone marrow biopsy showing metastatic colon adenocarcinoma. The tumour cells are forming cohesive sheets and there is occasional crypt and gland formation. Some cells also contain mucin. (c) Metastatic renal clear cell carcinoma. The tumour cells have clear abundant cytoplasm and could be confused with histiocytes or fat cells. There is nuclear hyperchromasia and pleomorphism. They are seen singly or in small clusters within an abundant fibrotic stroma. Paratrabecular aggregates are noted and there is bone remodelling. (d) The tumour cells are effacing the BM and are mostly showing well-formed glandular structure reminiscent to thyroid follicles. (e, f) Bone marrow biopsy showing metastatic prostate carcinoma. The tumour shows poorly differentiated areas and some areas with glandular differentiation. (Magnification: a–b ×20, c–f × 10)