Abstract
The classification of mature neoplasms has evolved over the years, with the current WHO classification based largely on the genetics and cellular origin of lymphoid neoplasms [1]. Clinical behaviour of lymphoid neoplasms, however, continues to play an important role in defining disease entities. Mature lymphoid neoplasms in the case of leukaemias primarily involve blood and bone marrow (BM), while in lymphomas, most entities, with an occasional exception (e.g. Waldenström macroglobulinaemia/lymphoplasmacytic lymphoma) show predominant involvement of extramedullary sites/site with secondary involvement of the bone marrow (BM). In patients with lymphomas, the BM may be biopsied as part of staging (e.g. follicular lymphoma) or when the primary site of involvement is not amenable to biopsy (e.g. primary splenic lymphomas such as splenic marginal zone lymphoma or hepatosplenic T-cell lymphoma). Less commonly, a mature lymphoid neoplasm may be an unexpected or suspected diagnosis initially made on BM biopsy (BMB) performed during investigation of B-symptoms or unexplained cytopaenias.
Introduction
The classification of mature neoplasms has evolved over the years, with the current WHO classification based largely on the genetics and cellular origin of lymphoid neoplasms [1]. Clinical behaviour of lymphoid neoplasms, however, continues to play an important role in defining disease entities. Mature lymphoid neoplasms in the case of leukaemias primarily involve blood and bone marrow (BM), while in lymphomas, most entities, with an occasional exception (e.g. Waldenström macroglobulinaemia/lymphoplasmacytic lymphoma) show predominant involvement of extramedullary sites/site with secondary involvement of the bone marrow (BM). In patients with lymphomas, the BM may be biopsied as part of staging (e.g. follicular lymphoma) or when the primary site of involvement is not amenable to biopsy (e.g. primary splenic lymphomas such as splenic marginal zone lymphoma or hepatosplenic T-cell lymphoma). Less commonly, a mature lymphoid neoplasm may be an unexpected or suspected diagnosis initially made on BM biopsy (BMB) performed during investigation of B-symptoms or unexplained cytopaenias.
The pattern of BM involvement (Table 15.1), cytological features and immunophenotype (+/– genetics) in conjunction, may be diagnostic or may help to narrow down the possibilities. Although the pattern of involvement is very useful, it is important to be aware that a particular pattern of BM involvement may be typical for an entity (e.g. paratrabecular involvement in follicular lymphoma), but is not specific and may be encountered in other lymphoid neoplasms.
Table 15.1 Patterns of bone marrow involvement encountered in mature lymphoid neoplasms.
| Pattern of involvement | Lymphoma subtypes | Comments |
|---|---|---|
| Focal/discrete, nodular, non-paratrabecular | CLL/SLL, MCL, SMZL, LPL, NMZL, MALT, DLBCL, HGBL, FL, B-PLL, AITL, PTCL NOS, ALCL, ATLL | Common pattern Distinction from benign aggregates may be difficult May be seen together with other patterns Seen rarely in FL usually when associated with neoplastic follicles |
| Focal/discrete, nodular, paratrabecular (infiltrate moulded against bony trabeculae) | FL, MCL, LPL, MALT, NMZL, AITL, PTCL NOS, ATLL | Virtually diagnostic of lymphoma as benign aggregates may abut but are not truly paratrabecular Typical of FL but may be seen in other lymphomas Not associated with CLL/SLL |
| Interstitial | HCL, CLL/SLL, SMZL, LPL, MCL, BL, SS, NMZL, MALT, B-PLL, ALCL, PTCL,NOS, T-LGLL, AITL, ATLL | Common pattern Often seen together with other patterns of involvement If subtle may require IHC for detection (e.g. ALCL) |
| Intrasinusoidal component | HSTL, intravascular LBCL, SDRPL, HCLv, SMZL, T-LGLL, ALCL, NMZL | May be overlooked on morphology and often requires IHC for detection Exclusive intrasinusoidal infiltrates feature of HSTL and intravascular LBCL; may also be seen in SDRPL and HCLv |
| Diffuse | HCL, DLBCL, HGBL, BL, CLL/SLL, MCL, FL, T-PLL, PTCL NOS | Seen in many lymphoma subtypes if there is heavy marrow involvement |
Abbreviations: CLL/SLL – chronic lymphocytic leukaemia/small lymphocytic lymphoma; MCL – mantle cell lymphoma; SMZL – splenic marginal zone lymphoma; LPL – lymphoplasmacytic lymphoma; NMZL – nodal marginal zone lymphoma; MALT– extranodal marginal zone lymphoma of mucosa associated lymphoid tissue; DLBCL – diffuse large B-cell lymphoma; HGBL – high grade B-cell lymphoma. FL – follicular lymphoma; B-PLL – B-prolymphocytic leukaemia; AITL – angioimmunoblastic T-cell lymphoma; PTCL NOS – peripheral T-cell lymphoma, not otherwise specified; HCL – hairy cell leukaemia; BL – Burkitt lymphoma, SS –Sézary syndrome; ALCL – anaplastic large cell lymphoma; T-LGLL – T-large granular lymphocytic leukaemia; HSTL – hepatosplenic T-cell lymphoma; intravascular LBCL – intravascular large B-cell lymphoma; SDRPL – splenic diffuse red pulp small B-cell lymphoma; HCLv – hairy cell leukaemia, variant; T-PLL – T-prolymphocytic leukaemia, ATLL – adult T-cell leukaemia/lymphoma. [2–9]
Mature B-Cell Neoplasms
These neoplasms range from the more indolent B-cell neoplasms, such as chronic lymphocytic leukaemia (CLL), to those that have variable clinical behaviour such as mantle cell lymphoma (MCL) and the more aggressive large cell lymphomas. In addition to the pace of disease, the sites of involvement play an equally important role in categorising these disorders, for example with the splenic and extranodal lymphomas. The typical morphological features associated with some of the leukaemic neoplasms, highlighted in the relevant sections, are best appreciated on a peripheral blood film and therefore blood films should ideally be examined together with other material.
Chronic Lymphocytic Leukaemia
Epidemiology and Clinical Features
As the commonest B-cell neoplasm in the western world, CLL is perhaps the most extensively studied lymphoid neoplasm and has well-defined immunomorphological characteristics (Table 15.2). The majority of cases are diagnosed incidentally on the basis of an abnormally high peripheral blood lymphocyte count performed for an unrelated reason. However, patients can present with lymphadenopathy, constitutional symptoms or BM failure. Small lymphocytic lymphoma (SLL) is essentially the same disease as CLL but is a term used for patients presenting with lymphadenopathy and less than 5 × 109/L circulating CLL phenotype cells.
Table 15.2 Immunogenetic profile of chronic lymphocytic leukaemia (CLL)/small lymphocytic lymphoma (SLL).
| CLL/SLL | ||
|---|---|---|
| Flow cytometric immunophenotypinga | Immunohistochemistry | Most common cytogenetic abnormalities |
| CD5+, CD19+, CD23+, CD43+ Weak CD20 and surface Ig CD22 and CD79b weak CD10–, FMC7– | Pan B-markers+, CD5+, CD23+, LEF1+, MUM1+ (in PCs) cyclinD1–, SOX11–, CD10– | Del 13q Tri 12 Del 11q Del 17p |
a Marsden score for CLL – CD5+, CD23+, SIg weak, CD79b (or CD22) weak, FMC7–. A score of ≥4 is compatible with a diagnosis of CLL [20].
Chronic lymphocytic leukaemia-related autoimmune cytopaenias are not uncommon and should be borne in mind when examining peripheral blood and bone marrow.
Monoclonal B-Lymphocytosis
Monoclonal B-cell lymphocytosis (MBL) is defined as a condition where the monoclonal B-cell count is less than 5 × 109/L in the absence of lymphadenopathy. One to two % of these cases have been observed to progress to CLL per year.
Monoclonal B-cell lymphocytosis is further divided into CLL-type MBL, where the lymphocytes bear the immunomorphological characteristics of CLL, and non-CLL MBL. A prerequisite to the diagnosis of MBL is the absence of clinical lymphadenopathy.
Peripheral Blood
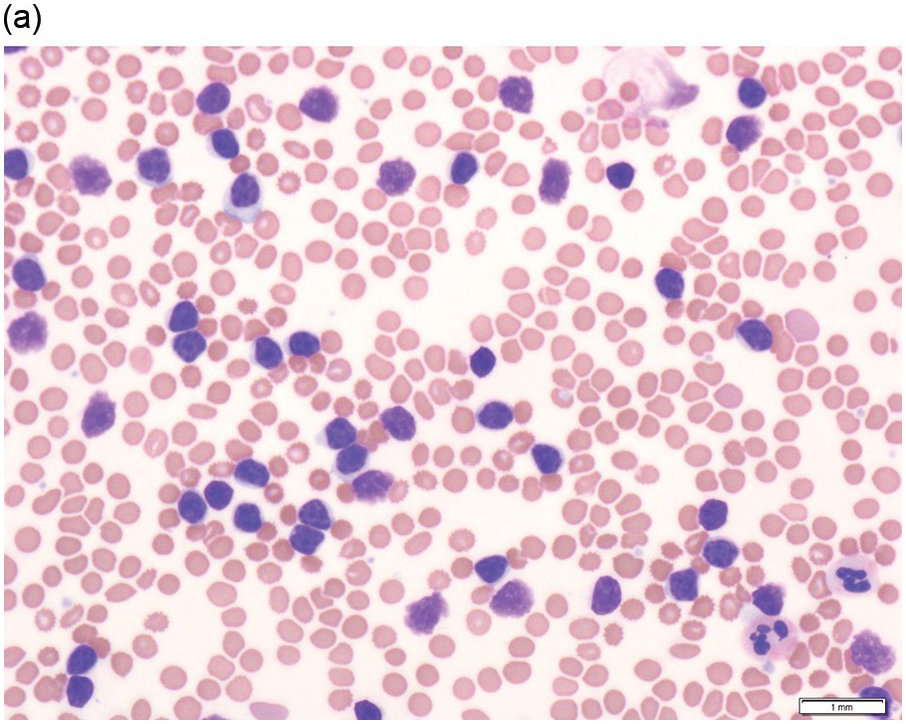
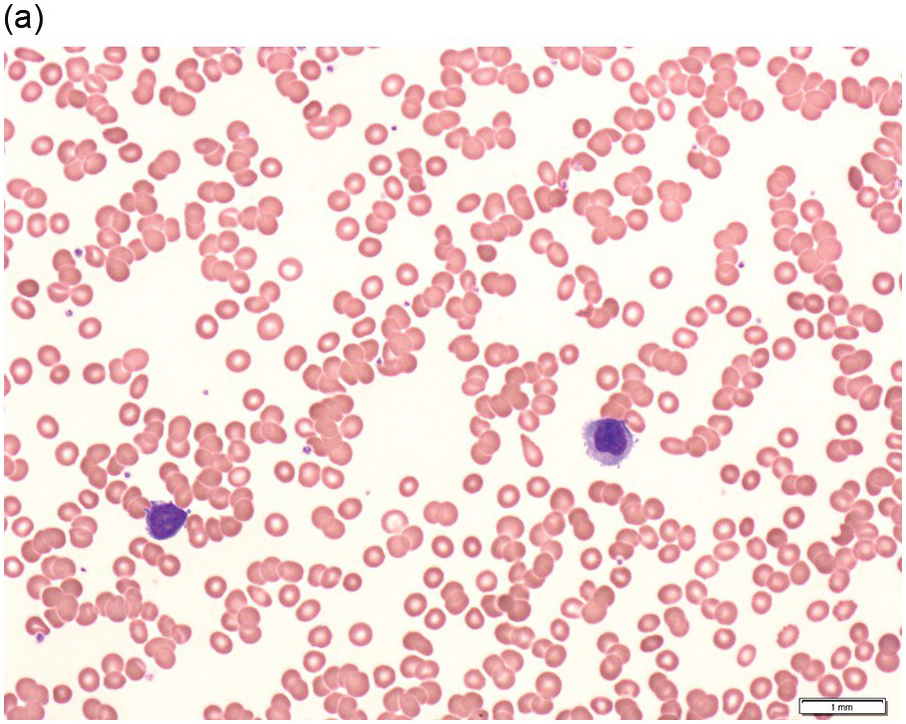
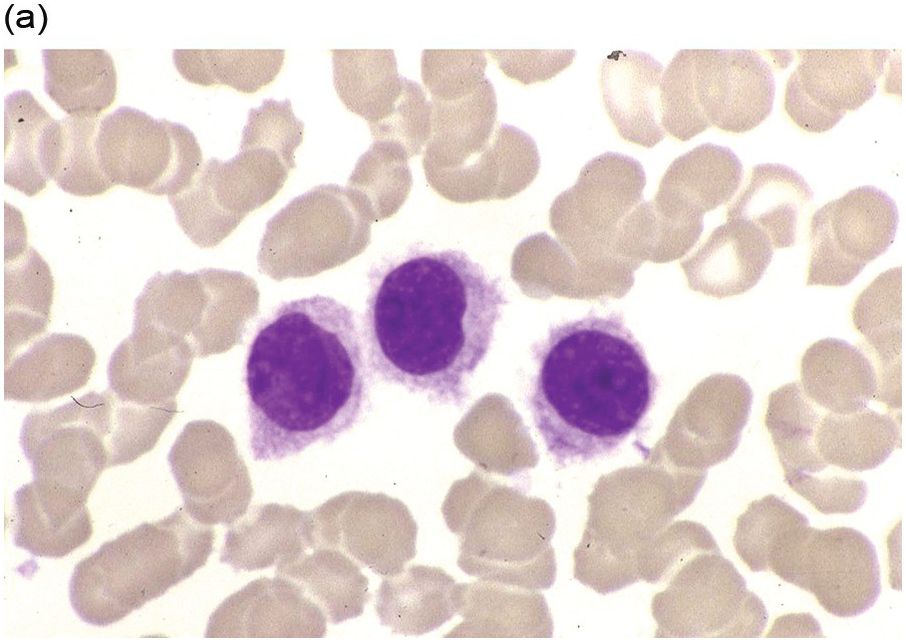
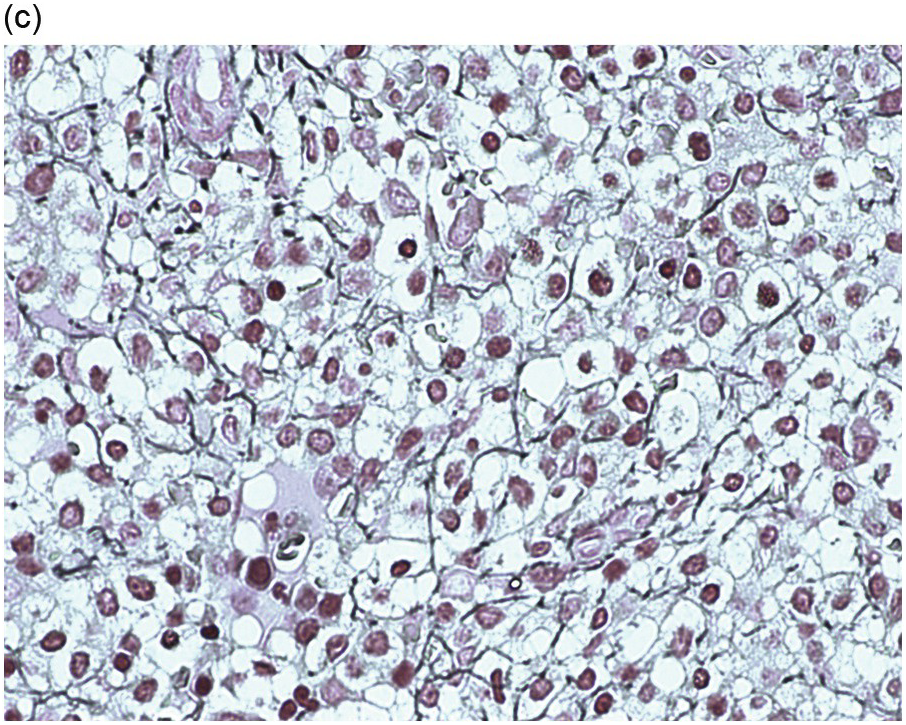
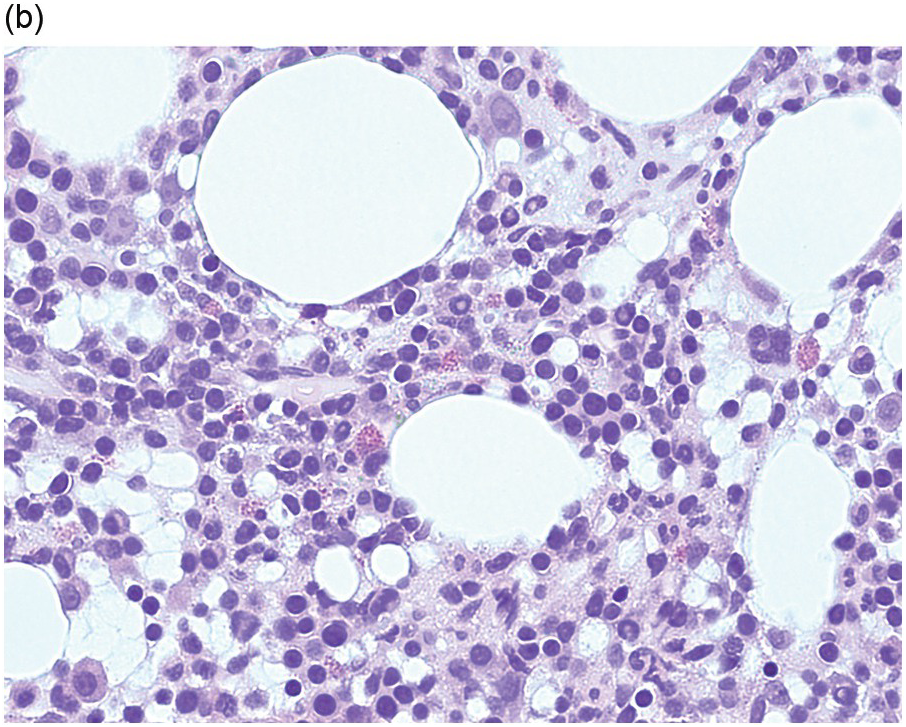
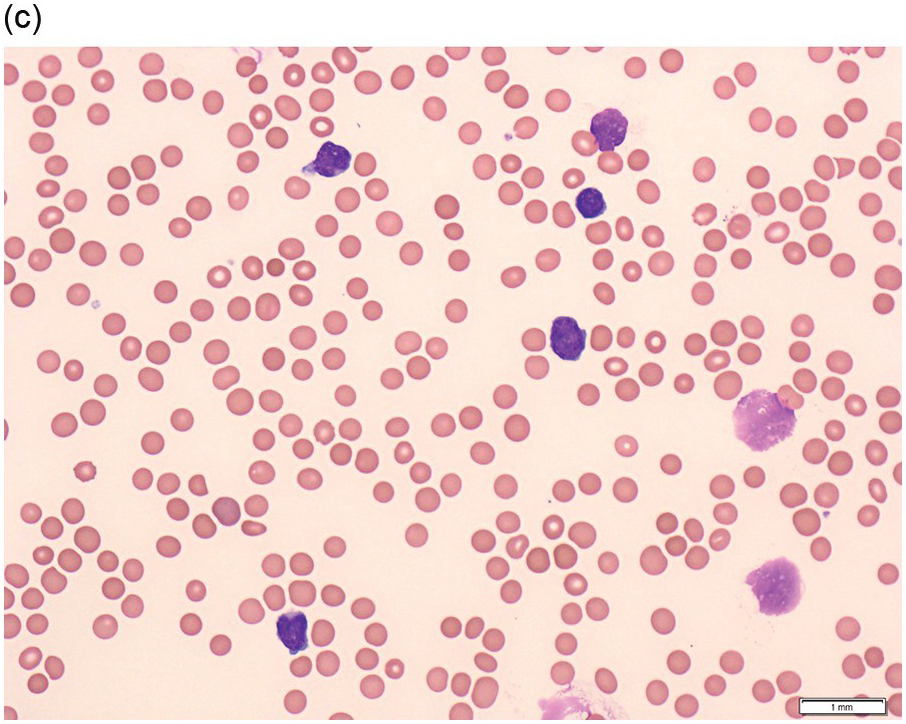
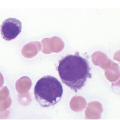
The peripheral blood typically shows a marked increase in small, mature lymphocytes that have scanty cytoplasm and clumped nuclear chromatin (Figure 15.1a). The nuclear chromatin in CLL has often been described as having a ‘cracked-mud’ appearance. In addition to these cells that are typical for CLL, there are other cells with atypical morphology, including prolymphocytes and/or cells with more abundant cytoplasm and/or indented nuclei. The threshold of ≥15% of such circulating atypical cells has been proposed to define CLL with atypical morphology [10]. Prolymphocytes should not exceed 55%. Smudge cells (also known as basket cells) are not diagnostic of CLL but are frequently seen in the peripheral blood.
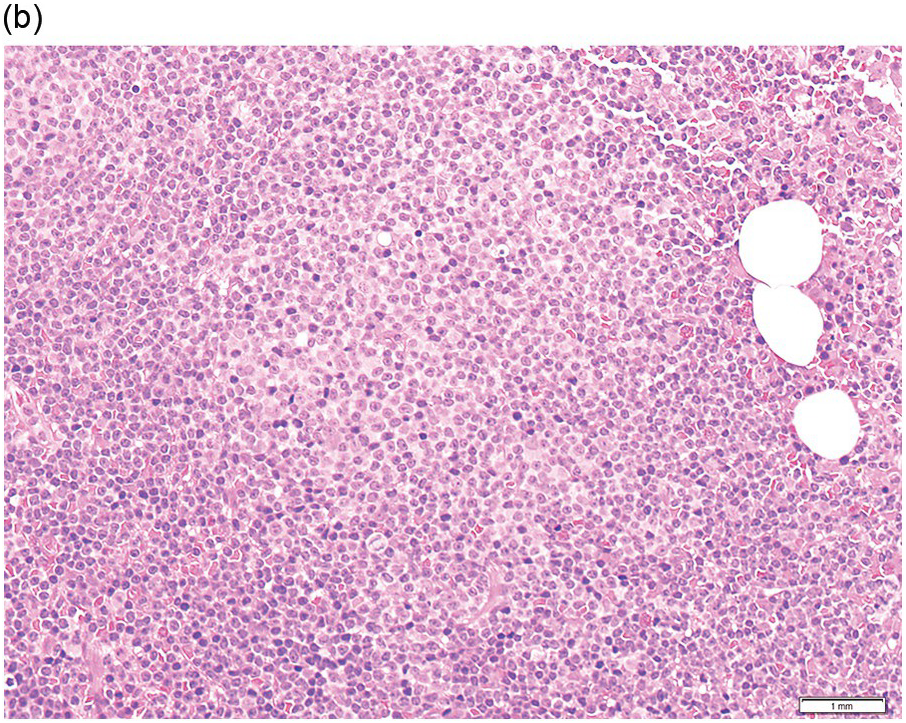
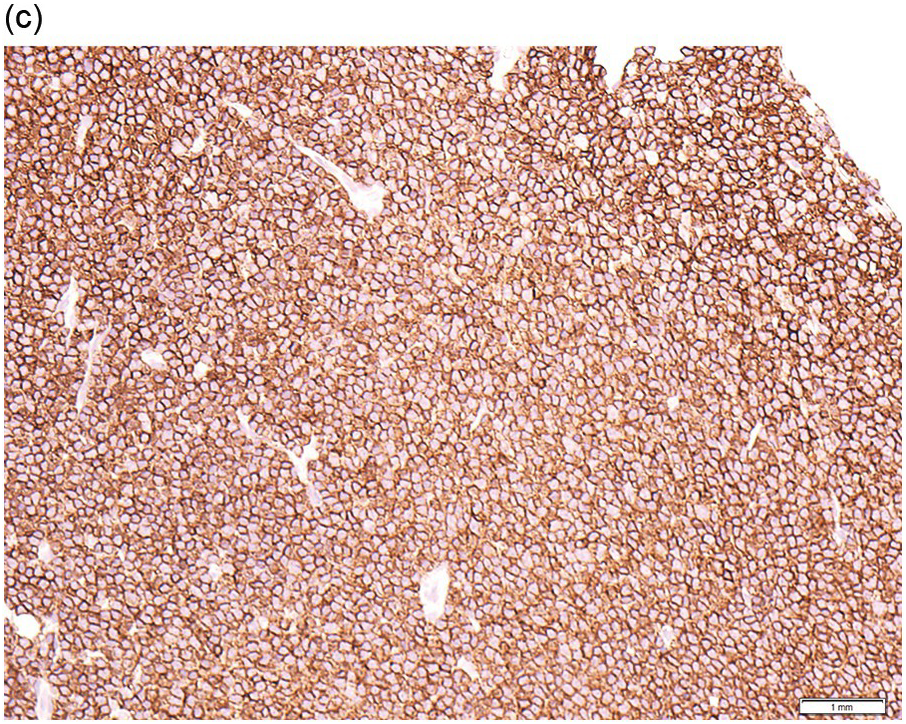
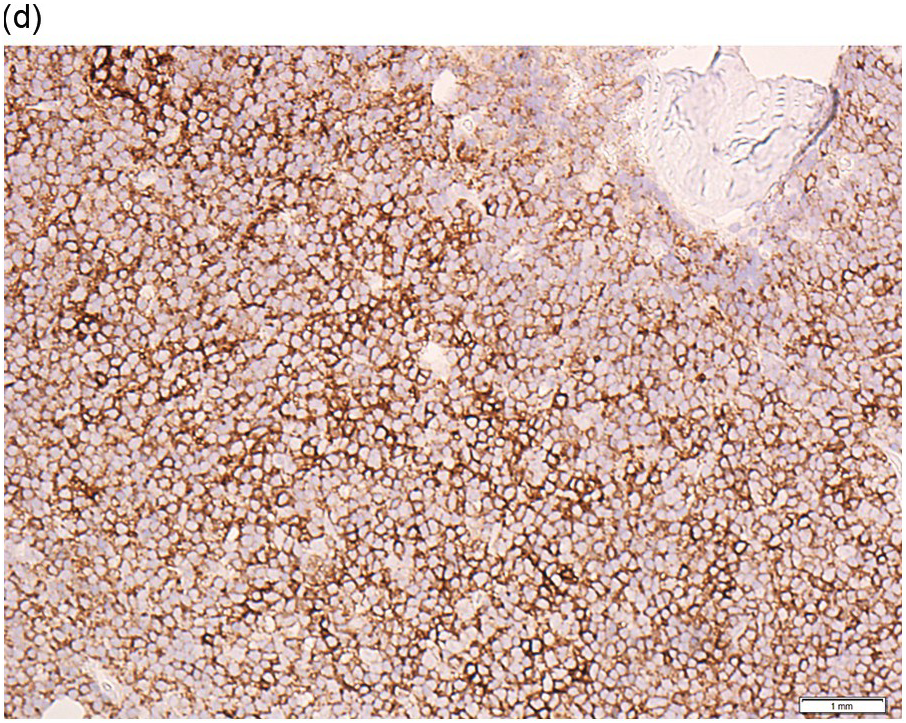
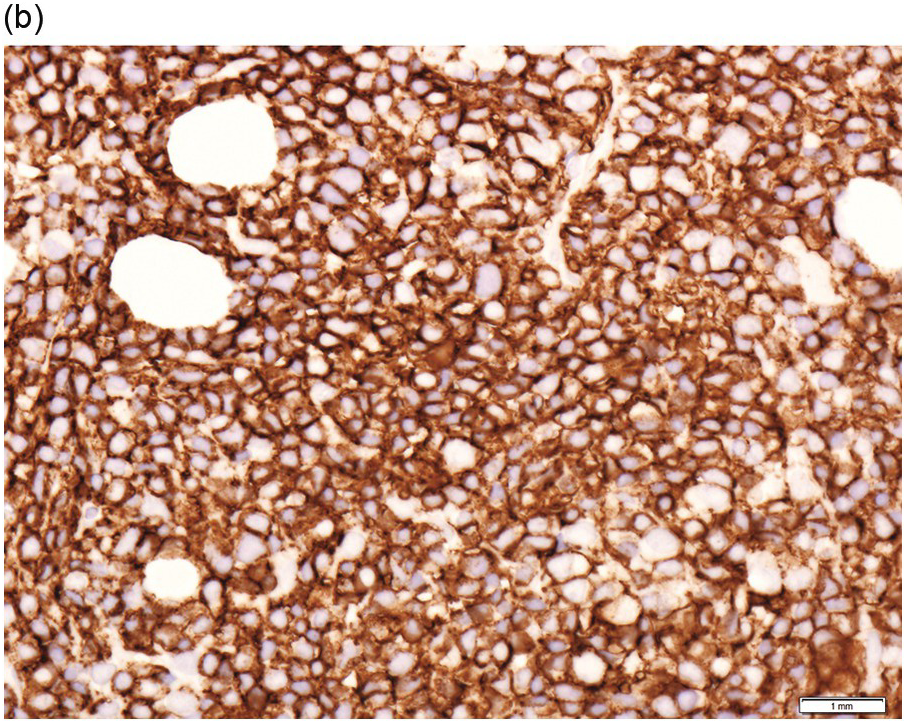
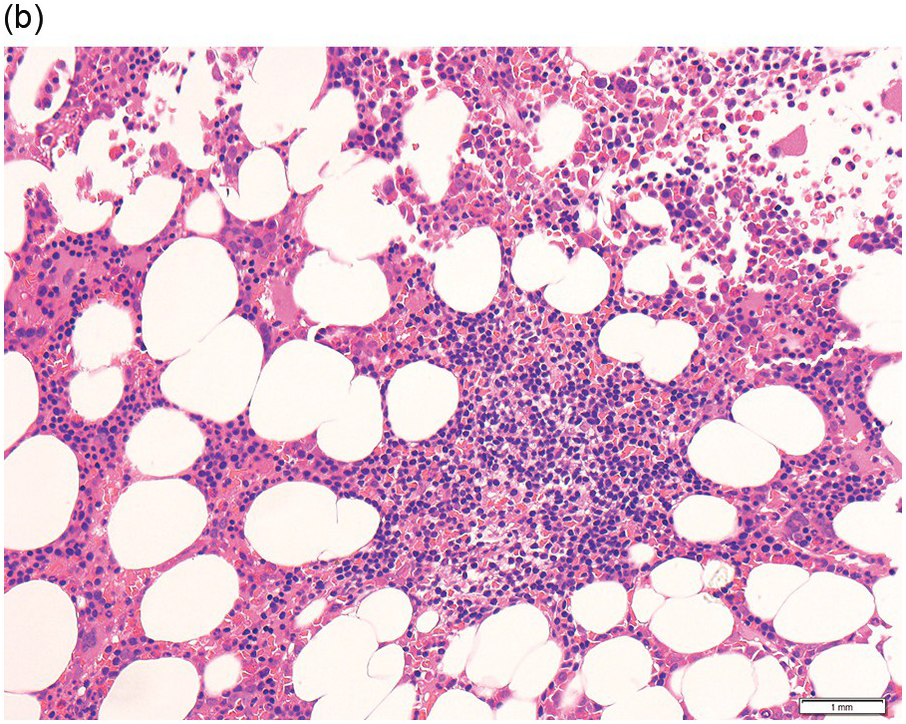
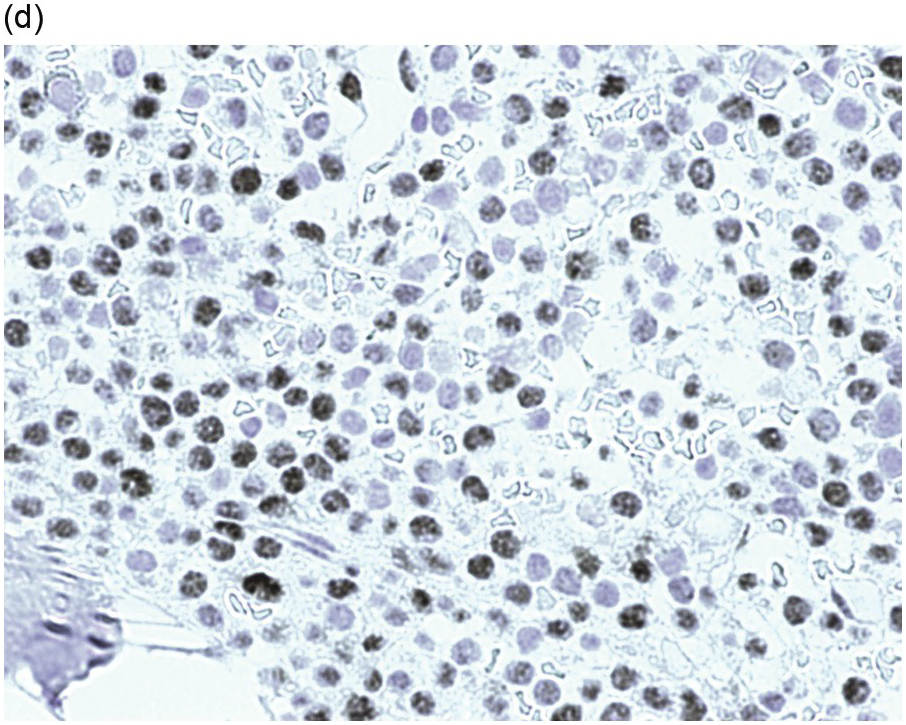
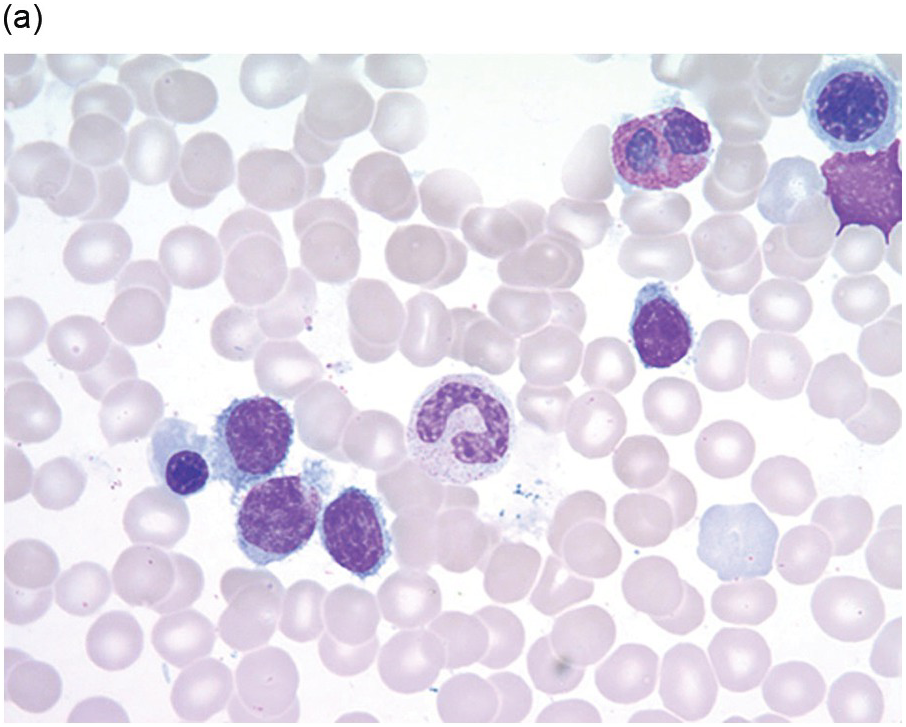
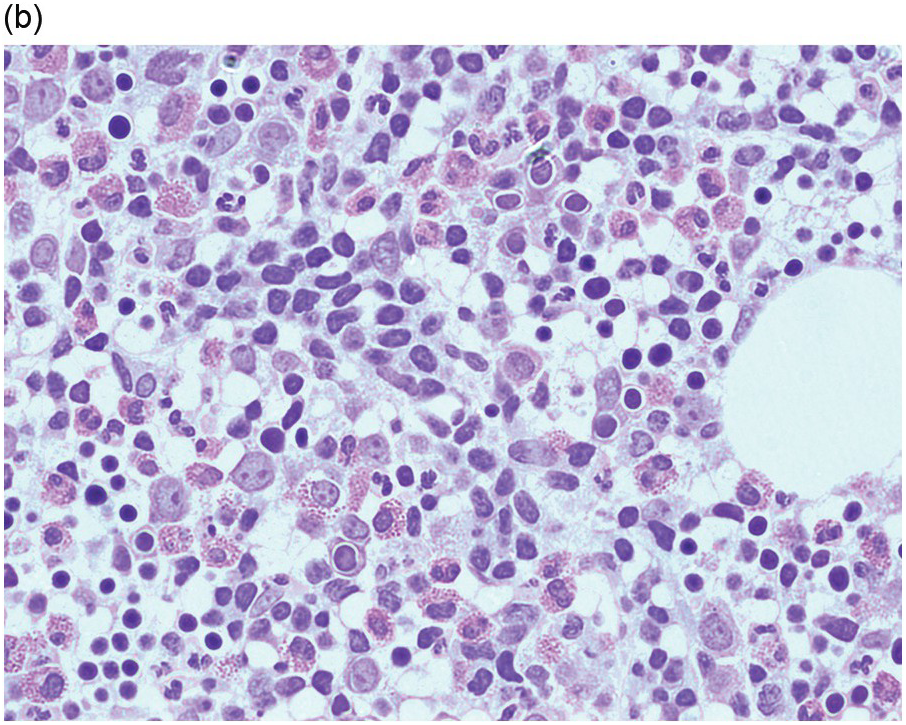
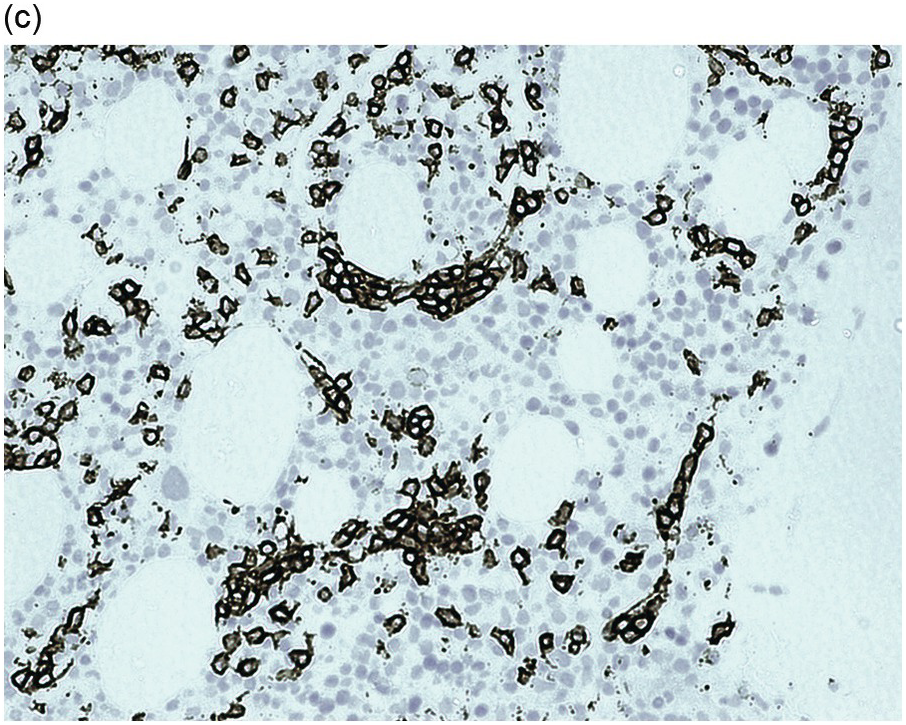
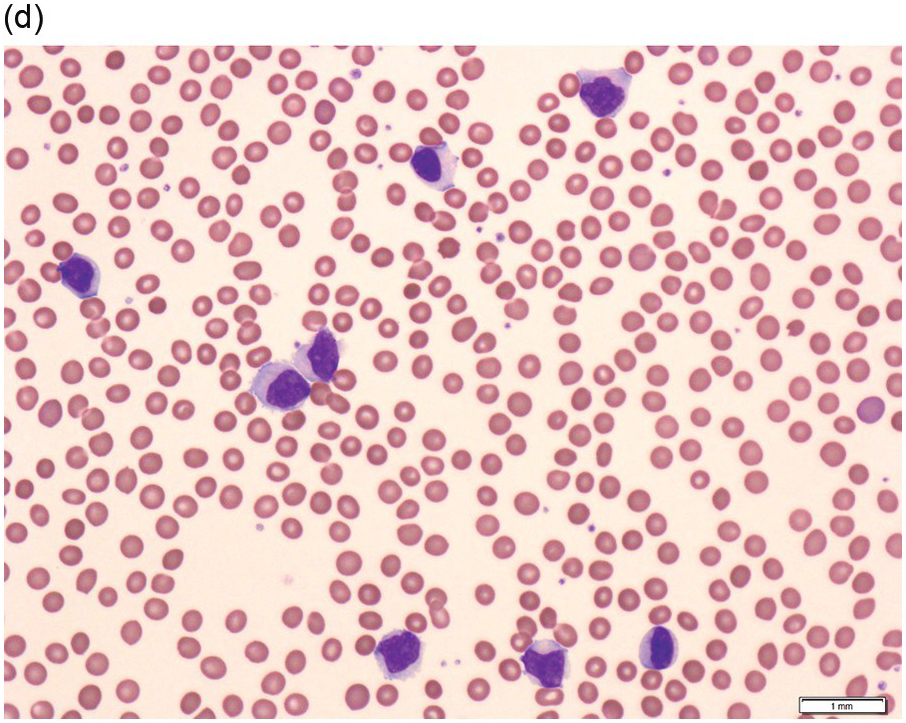
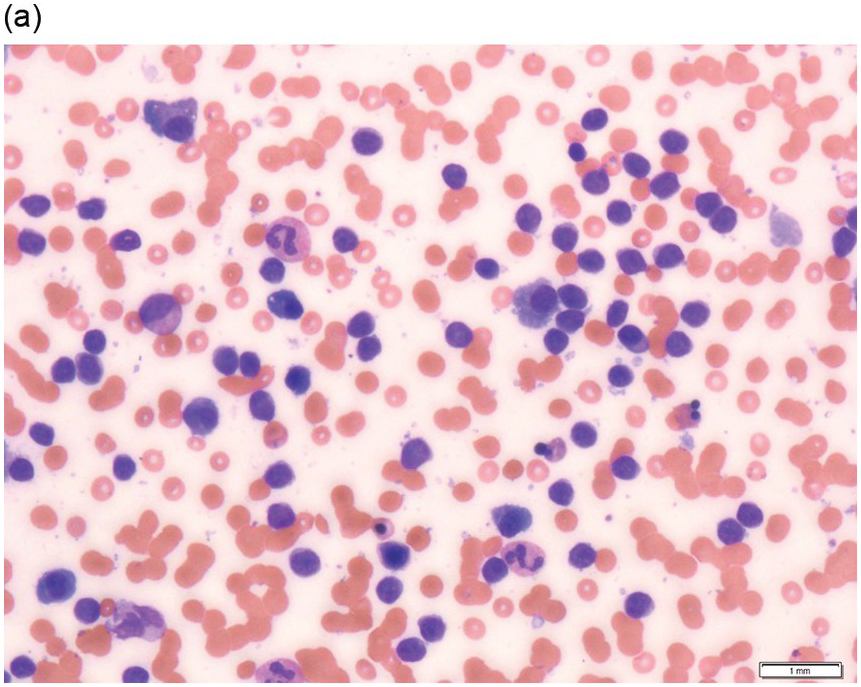
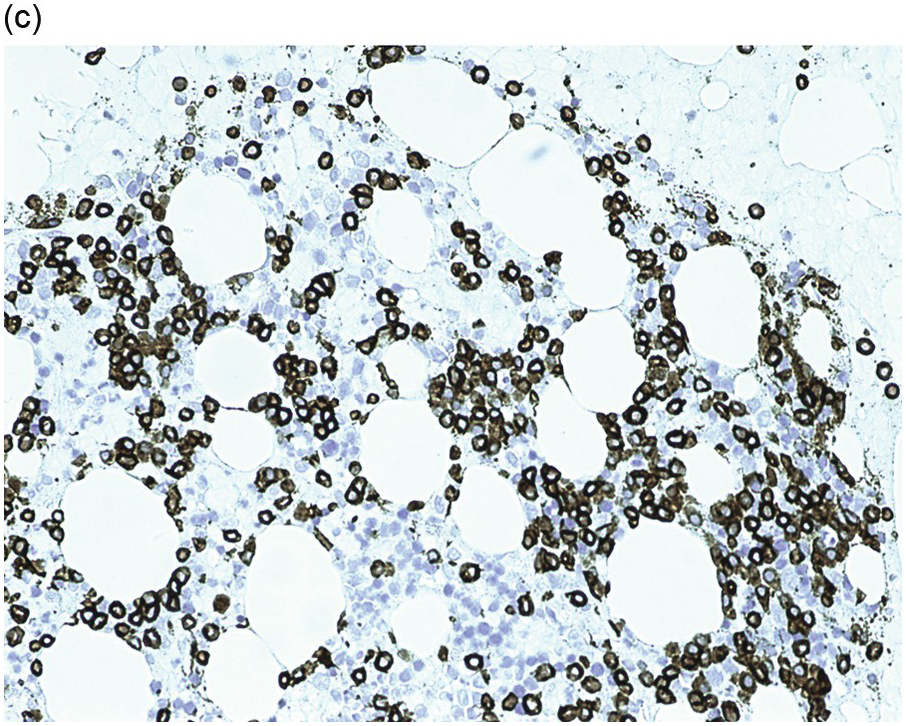
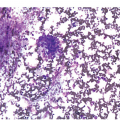
Figure 15.1 Chronic lymphocytic leukaemia (CLL). (a) Peripheral blood film showing small mature lymphocytes. (b–d) Pre-treatment bone marrow trephine biopsy. Haematoxylin and eosin (H&E) stained section shows the nodular and interstitial infiltrate with a prominent proliferation centre (b). The infiltrate is positive for CD5 (c) and CD23 (d).
Bone Marrow Aspirate and Biopsy
BM aspirate and biopsy are not usually performed in early-stage disease if the patient is to be managed expectantly, but are recommended as baseline tests prior to commencing treatment and are important in the assessment of response to therapy. They may also be informative when autoimmune cytopaenia is suspected.
Bone Marrow Aspirate
Pre-treatment/untreated bone marrow aspirate in CLL/SLL shows a typically hypercellular bone marrow with a monomorphic infiltrate of mature lymphocytes and a reduction in myeloid and erythroid precursors. The degree of infiltration may not correspond to the blood counts in that patients with normal blood counts can have heavy CLL infiltration and vice versa. The possibility of peripheral consumption/destruction should be borne in mind when assessing normal haematopoietic precursors in the aspirate.
Post-Treatment Bone Marrow Aspirate
A normocellular aspirate with no evidence of CLL is among the criteria required to define a complete response post-treatment as per the 2018 iwCLL guidelines [11].
Bone Marrow Biopsy
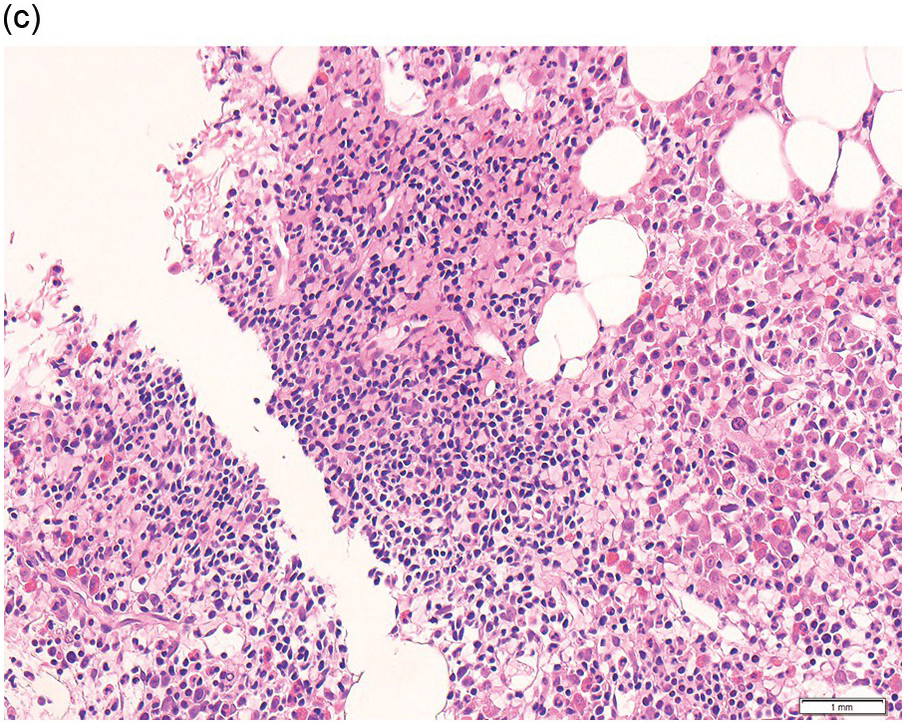
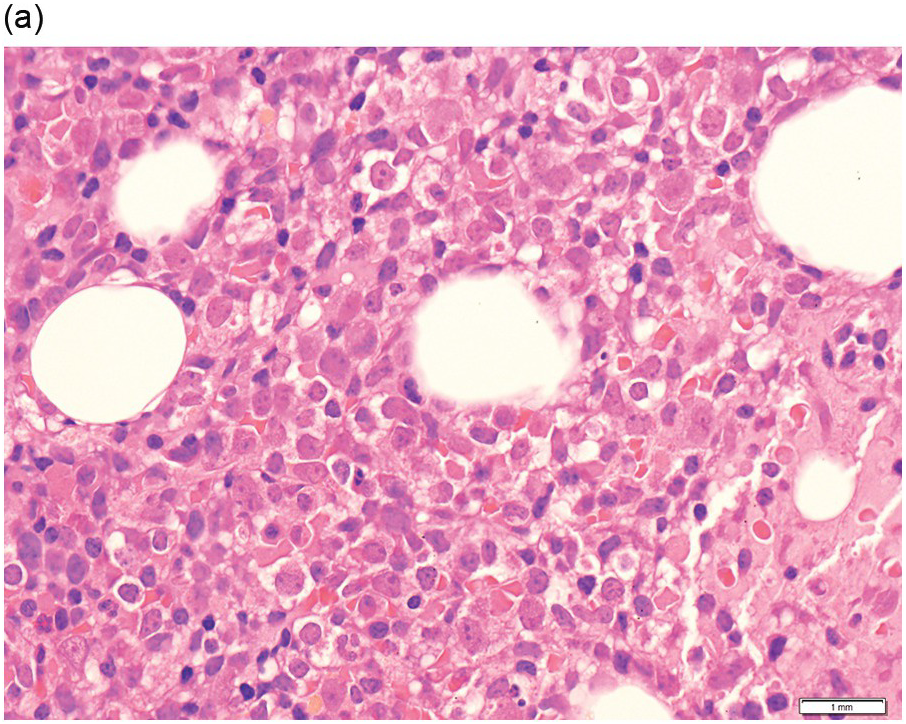
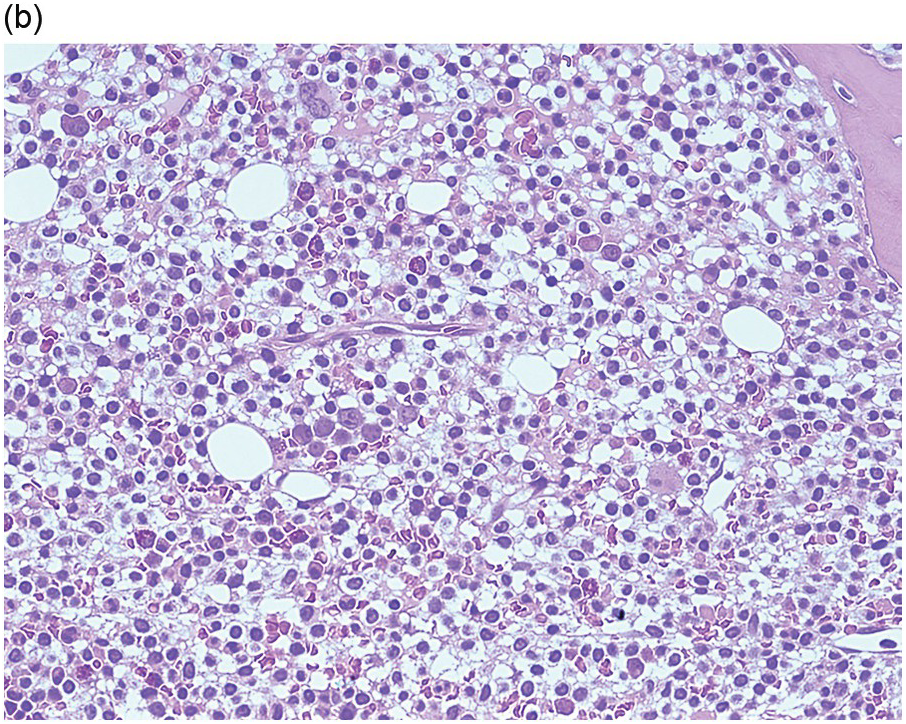
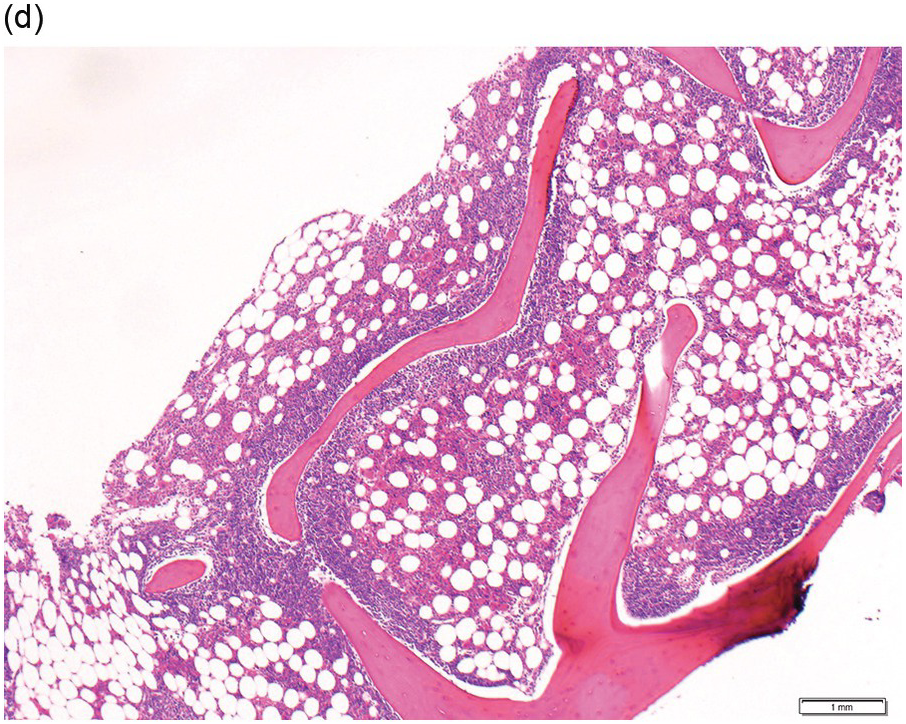
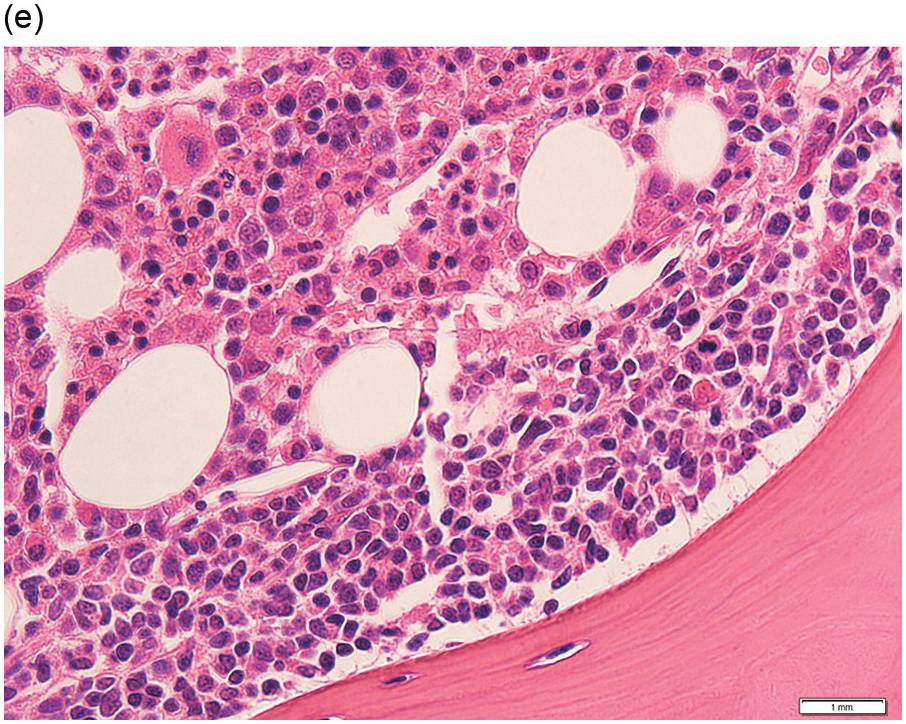
Pre-treatment/untreated bone marrow biopsy in CLL/SLL usually shows a nodular (not usually paratrabecular) and interstitial pattern of infiltration (Figure 15.1b) amid which are reduced residual islands of erythropoiesis and myelopoiesis, and often relative preservation of megakaryocytes. If the infiltrate is very heavy the BM spaces may be packed with a diffuse infiltrate that replaces haematopoietic elements and fat spaces.
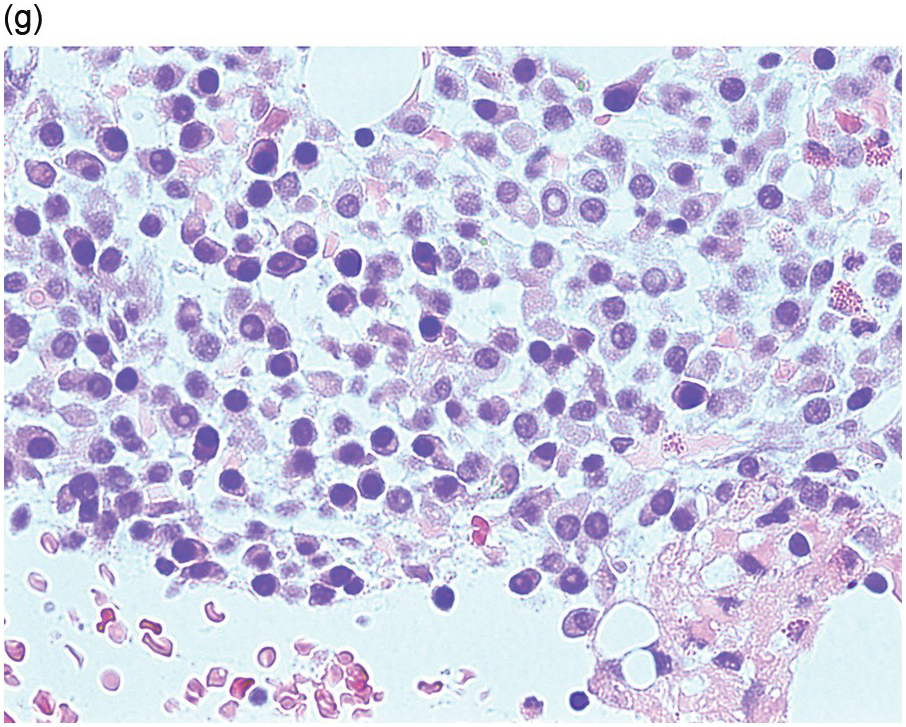
The infiltrate is composed predominantly of small regular mature lymphocytes. Scattered prolymphocytes (medium-sized cells with clumped nuclear chromatin and a small nucleolus) may be seen amid the small lymphocytes, but prolymphocytes and larger paraimmunoblasts (large cell with dispersed nuclear chromatin and a distinct central eosinophilic nucleolus) may be concentrated to form nodular proliferation centres (Figure 15.1b). The latter are unique to CLL and are not a feature of any other lymphoid neoplasm [12].
Post-Treatment Bone Marrow Biopsy
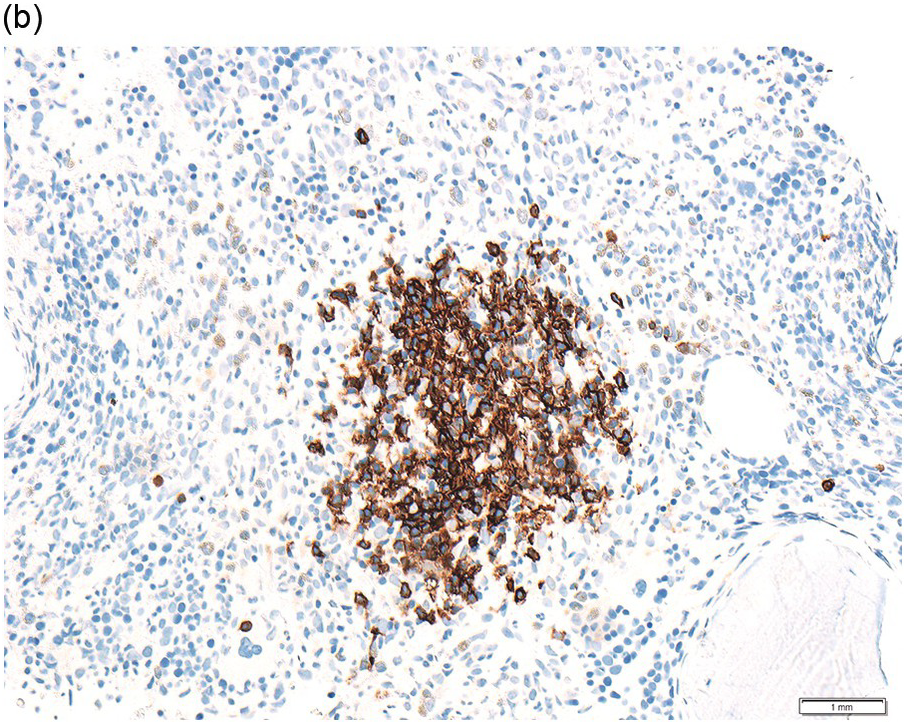
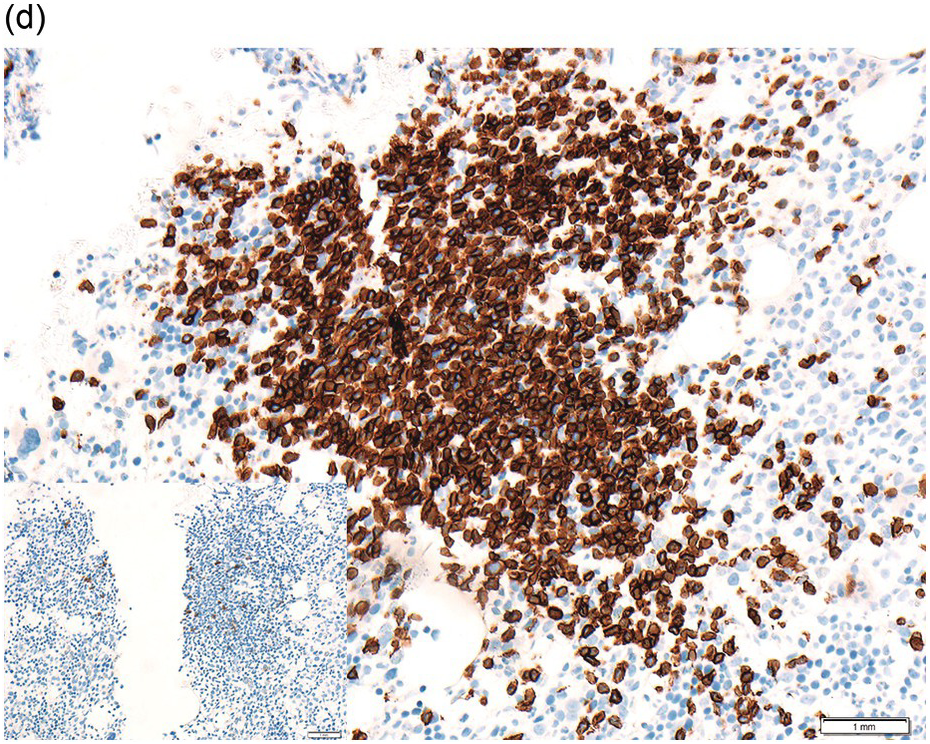
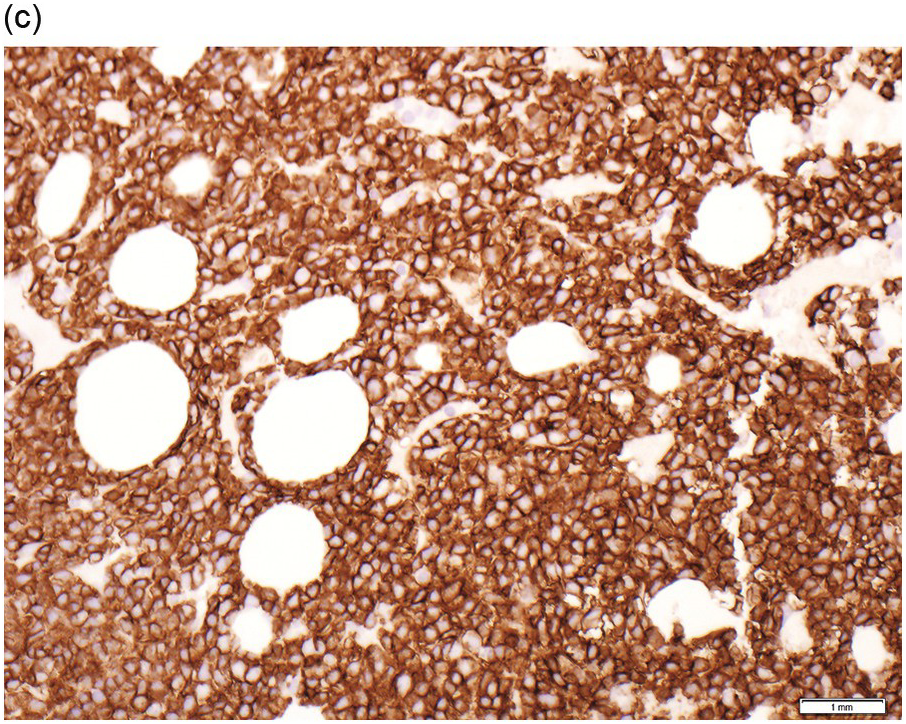
The interstitial component decreases and the residual lymphoid infiltrate may be mainly or exclusively nodular. These nodules are associated with a localized increase in reticulin and therefore if the infiltrate is entirely nodular and sparse it may not be represented in the BM aspirate sample. It is also imperative that immunohistochemistry be used to evaluate the nodular infiltrates, to distinguish between reactive T-cell nodules and neoplastic B-cells (Figure 15.2). There is often an increase in eosinophils surrounding the T-cell aggregates [12]. Please see Chapter 18 for chemotherapy-related BM changes.
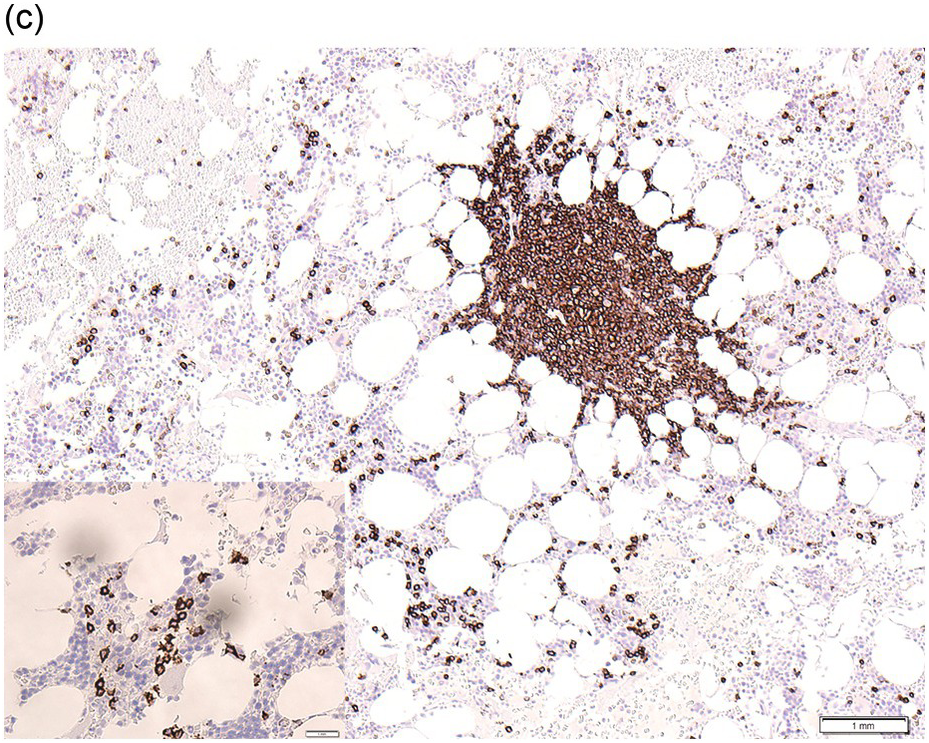
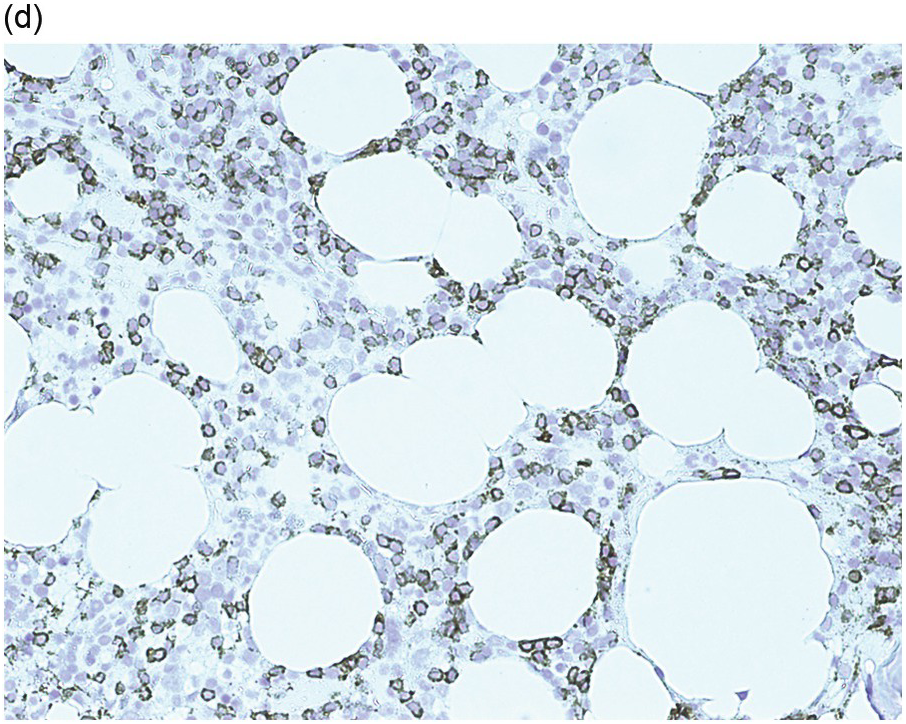
Figure 15.2 Bone marrow trephine biopsy, post-treatment. (a, b) B-cell nodule. H&E stained section shows eosinophils surrounding a nodule of small lymphoid cells (a), composed of numerous CD20 positive B-cells (b). (c, d) A predominantly T-cell nodule. H&E stained section shows a nodule of small lymphoid cells (c) that is composed predominantly of CD3 positive T-cells (d) with only a few scattered CD23 positive B-cells (d, inset).
Immunohistochemistry
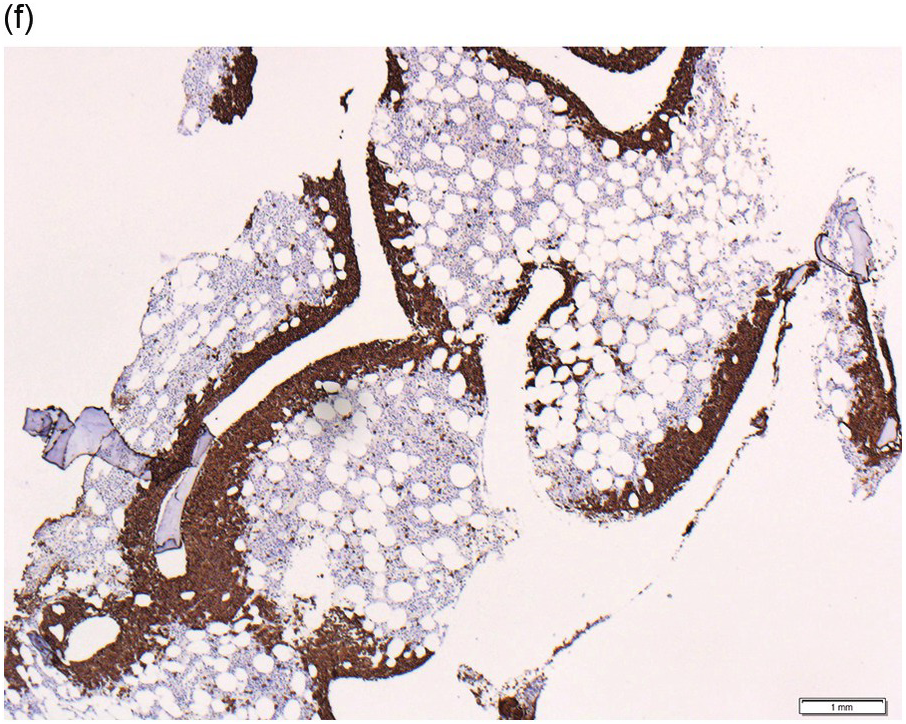
The immunoprofile (summarized in Table 15.2) of CLL is usually determined by flow cytometry and therefore immunohistochemistry (IHC) does not usually play an important part in its characterization (Figure 15.1c, d). The neoplastic infiltrate expresses pan B-markers CD20, CD79a, CD19 and PAX5 and also co-expresses CD5, the latter often shows a staining intensity weaker than that of background T-cells. CD23 expression often varies in intensity with strong expression in prolymphocytes and paraimmunoblasts and therefore in proliferation centres (Figure 15.1) [12]. Aberrant expression of LEF1 is fairly specific for CLL as it is negative in normal mature B-cells and negative (with the exception of rare cases) in other small B-cell neoplasms [13]. Proliferation centres are highlighted by MUM1. SOX11 is negative and cyclin D1 is usually negative, but in a minority of cases there may be a few weakly cyclin D1 positive cells in proliferation centres [14]. MYC and NOTCH1 proteins may also be expressed in CLL, irrespective of genetic abnormalities [15, 16]. Immunohistochemistry is imperative in distinguishing the composition of lymphoid nodules post chemotherapy, to distinguish between reactive T-cell nodules and neoplastic infiltrates (Figure 15.2).
As in the case of other B-cell neoplasms treated with anti-CD20, B-markers, such as CD79a, CD19 or PAX5, instead of CD20 are recommended to assess for residual involvement.
Haematogones seen in regenerating BM are highlighted by CD79a or PAX5 and even if florid and loosely clustered should not cause confusion, as they have a rather polymorphous appearance, with a proportion expressing TdT and unlike in CLL they are negative for CD5 and CD23 [12].
Genetics
The common cytogenetic abnormalities are listed in Table 15.2. TP53 mutations, including small subclones, predict poor response to immunochemotherapy and should therefore be routinely tested for prior to therapy [17, 18]. Mutated immunoglobulin heavy chain genes (IGHV) in CLL are associated with significantly better outcomes in the context of immunochemotherapy, compared to unmutated IGHV [17]. CD38 and ZAP70 positivity is a surrogate marker for unmutated IGHV in CLL but is less reliable than molecular testing of IGH genes.
Next-generation sequencing studies in CLL have identified recurrent mutations in genes such as NOTCH1, SF3B1, TP53, ATM and BIRC3 [19]. Although testing for these mutations is not widely available, some of these mutations are associated with inferior response/resistance to certain therapies. One such example is the resistance to rituximab seen in NOTCH1 mutated CLL [17].
The term CLL with atypical immunophenotype has been used in cases where the immunoprofile is somewhat inconsistent with a diagnosis of CLL/SLL. When employing the Marsden CLL score [20], for example, these cases often score less than 3 out of 5. Cases of trisomy 12 and a rarer subset harbouring t(14:19) or the IGH/BCL3 translocation often fall in this category [21].
Progression and Transformation
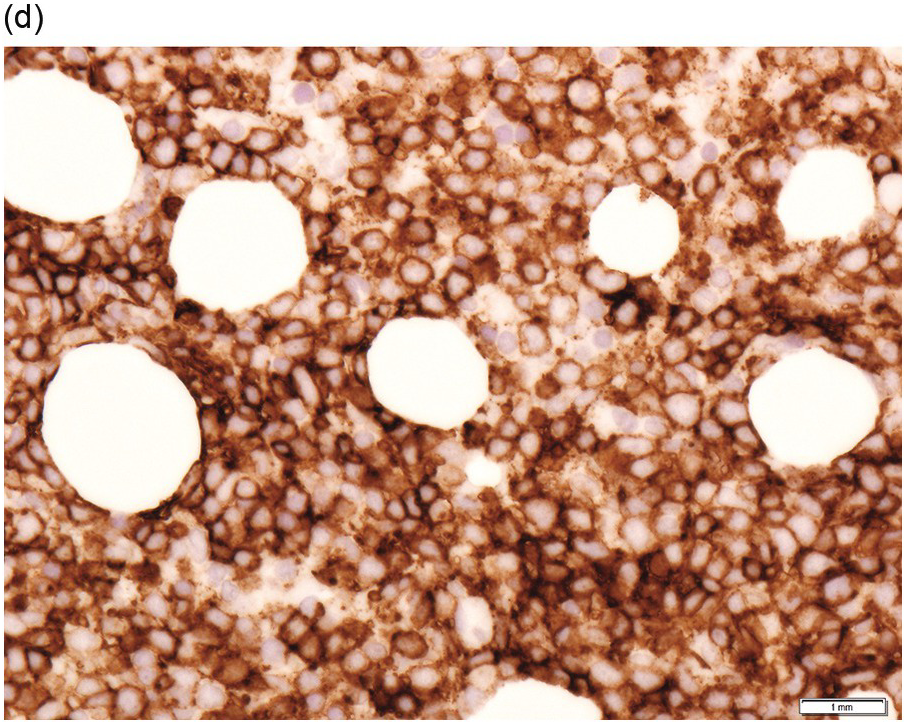
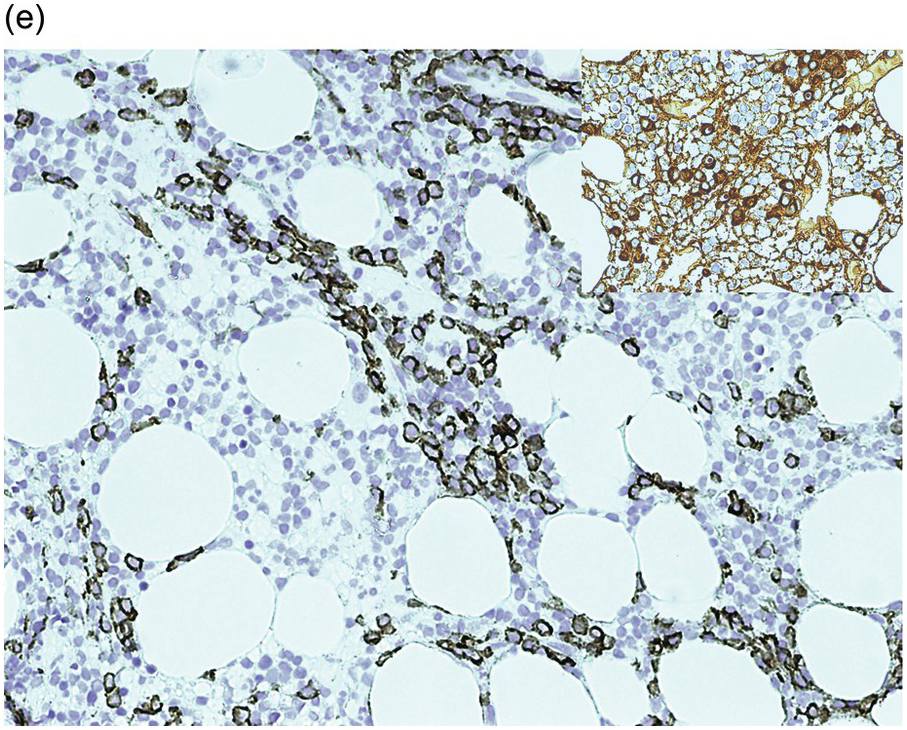
Chronic lymphocytic leukaemia/prolymphocytic leukaemia (CLL/PLL) is defined by >10 and <55% prolymphocytes/paraimmunoblasts in the peripheral blood and has a more aggressive clinical course. With progression, an increased number of prolymphocytes (≥55% of total lymphocytes) is seen in the peripheral blood (prolymphocytoid transformation) [1]. Histological progression in the form of expanded and coalescing proliferation centres is encountered in lymph nodes, while the BM may show prominent proliferation centres or a variable increase in prolymphocytes/paraimmunoblasts distributed amid small mature B-cells [22, 23]. The development of diffuse large B-cell lymphoma (DLBCL) in a patient with CLL is referred to as Richter transformation (Figure 15.3) and has an estimated incidence of 5 to 10%. In most cases, the DLBCL is clonally related to the CLL. In this instance, the median survival is <1 year whereas in those cases with a DLBCL unrelated to the CLL, the DLBCL prognosis is similar to that of de novo DLBCL. The transformation is often detected at biopsy of an extramedullary site while the BM still shows CLL, but in a few cases the initial diagnosis of DLBCL is made on BMB. Rarely, classical Hodgkin lymphoma (cHL) may develop in patients with CLL and requires the presence of diagnostic Hodgkin–Reed–Sternberg (HRS) cells in an appropriate inflammatory background for diagnosis. Classical Hodgkin lymphoma must be distinguished from HRS cells in CLL (which does not fulfil the criteria for cHL).
Figure 15.3 Richter transformation to diffuse large B-cell lymphoma (DLBCL). H&E stain shows the diffuse sheet of large atypical lymphoid cells (a), that are positive for CD20 (b), CD5 (c) and CD23 (d).
B-Prolymphocytic Leukaemia
B-prolymphocytic leukaemia (B-PLL) is an extremely rare entity and remains a diagnosis of exclusion. Patients typically present with a high white cell count, circulating prolymphocytes (>55%) and splenomegaly. An important differential diagnosis is MCL and this should be excluded by FISH for IGH/CCND1 [24, 25]. Please refer to Table 15.3 for salient clinical features, pattern of splenic involvement, peripheral blood, BMB morphology, immunoprofile and genetics.
| aB-cell prolymphocytic leukaemia | Hairy cell leukaemia | Splenic marginal zone lymphoma | Splenic diffuse red pulp small B-cell lymphoma | Hairy cell leukaemia variant | |
|---|---|---|---|---|---|
| Salient clinical features in addition to splenomegaly | High WCC B-symptoms Cytopaenias | Pancytopaenia Monocytopaenia Fatigue Infections Hepatomegaly | Autoimmune cytopaenias Cold AIHA Hepatitis C | Low level lymphocytosis | High WCC Normal monocyte count |
| Pattern of splenic involvement | Infiltration of white pulp and red pulp | Diffuse red pulp infiltration with atrophic white pulp; red cell lakes often seen | Characteristic nodular expansion of white pulp (inner zone of small cells that surround/replace GCs and outer zone of cells with more abundant cytoplasm); red pulp is always infiltrated | Diffuse infiltration of red pulp; blood lakes may be seen. White pulp involvement is absent and often atrophic | Diffuse infiltration of red pulp with atretic or absent white pulp follicles |
| Peripheral blood | Prolymphocytes – medium sized with a single prominent nucleolus >55% of lymphoid cells | Lymphocytes with oval/indented nuclei and ‘hairy’ cytoplasmic projections | Mature lymphocytes with polar cytoplasmic villi | Villous lymphocytes | Prolymphocytes with hairy cytoplasmic projections |
| Bone marrow trephine | Histology is not specific and plays a supportive role in diagnosis. Interstitial or intertrabecular nodular infiltrates | Interstitial or diffuse; nodular involvement not a feature. Neoplastic cells impart a ‘fried egg’ appearance. Increase in marrow reticulin (see text for detailed description) | Nodular, interstitial and intrasinusoidal infiltration. Nodules mostly intertrabecular, but may be paratrabecular. Nodular infiltrates may contain reactive GCs (see text for detailed description) | Predominantly and may occasionally be exclusively intrasinusoidal. IHC may be required to highlight the infiltrate. May also have an interstitial or nodular component. Follicles not reported | Infiltrates may be subtle and require IHC for detection. Infiltrates interstitial and/or intrasinusoidal. Latter may be prominent |
| Immunophenotype | SmIg strong, CD19+, CD79a+, CD20+, CD22+, FMC7+, CD5+/–CD23– | CD19+, CD20+, CD22+, CD200+, annexin A1+ CD5 Typically all 4 below expressed: CD11c+, CD25+, CD103+, CD123+ | CD19+, CD20+, CD22+, FMC7+, CD5+/–, CD23+/–, annexin A1– CD11c+/–, CD25+/–, CD103 and CD123 usually not expressed | CD19+, CD20+, CD22+, FMC7+, CD5+/–, CD11c+/–, annexin A1– | CD19+, CD20+, CD22+, annexin A1– CD11c+, often CD103+, CD25 and CD123 usually not expressed |
| Genetics | 17p deletion in 50% of cases | BRAF V600E mutation in most cases. Absent in IGHV4-34 rearranged classic HCL cases and in HCL variant. | 7q– in 30% NOTCH2 mutated in 10–25% cases. KLF2 mutated 10–40% cases. Evidence limited but NOTCH2, KLF2 and TP53 mutations associated with adverse prognosis | Recurrent CCND3 mutations | MAP2K1 mutations in up to 50% of cases |
Splenic Marginal Zone Lymphoma (SMZL)
Marginal zone lymphomas (MZL) originate from post-germinal centre B-cells. They are divided according to their primary site of proliferation into splenic, extranodal mucosa associated lymphoid tissue (MALT) or nodal subtypes (please see below for sections on MALT lymphoma and nodal MZL).
Epidemiology and Clinical Features
Splenic marginal zone lymphoma is a rare neoplasm (<2% of lymphoid neoplasms) that usually occurs after the fifth decade of life though it is occasionally seen in younger patients. Cytopaenias and splenomegaly are common presenting features. Important associations include autoimmune disease and hepatitis C infection (Table 15.3).
Blood and Bone Marrow Aspirate
A majority of patients have circulating polymorphic appearing mature lymphocytes, some of which have villous cytoplasmic projections at one pole (polar villi) (Figure 15.4a). Lymphoplasmacytoid cells may also be seen. The nuclei of SMZL cells have condensed chromatin and may display a small nucleolus.
(a) peripheral blood film showing mature lymphoid cells with polar villi. (b, c) Bone marrow trephine biopsy.
(b) H&E stained section highlights the nodular infiltrate.
(c) CD20 stain shows the nodular infiltrate and also highlights interstitial and intrasinusoidal (inset) not appreciated on H&E stain.
BM aspirate cytology can be normal in SMZL with no obvious excess of lymphocytes and infiltration, when present, is often not heavy. Splenic marginal zone lymphoma lymphocytes have mature appearances and lymphoplasmacytoid features are sometimes seen. Flow cytometric immunophenotyping can pick up small BM SMZL clones in the majority of cases.
Bone Marrow Biopsy
Diagnosis is best made on splenectomy but BMB is very rarely performed especially early in the course of the disease. Therefore BMB appearances in conjunction with clinical findings, imaging, blood and aspirate morphology, immunoprofile, genetic and cytogenetic abnormalities are often relied upon for diagnosis.
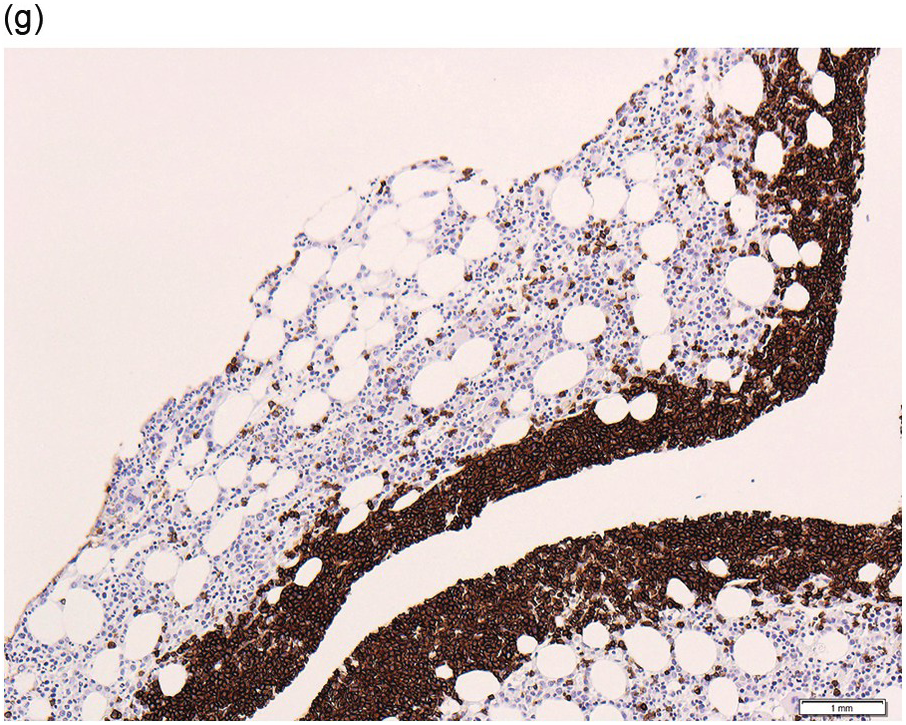
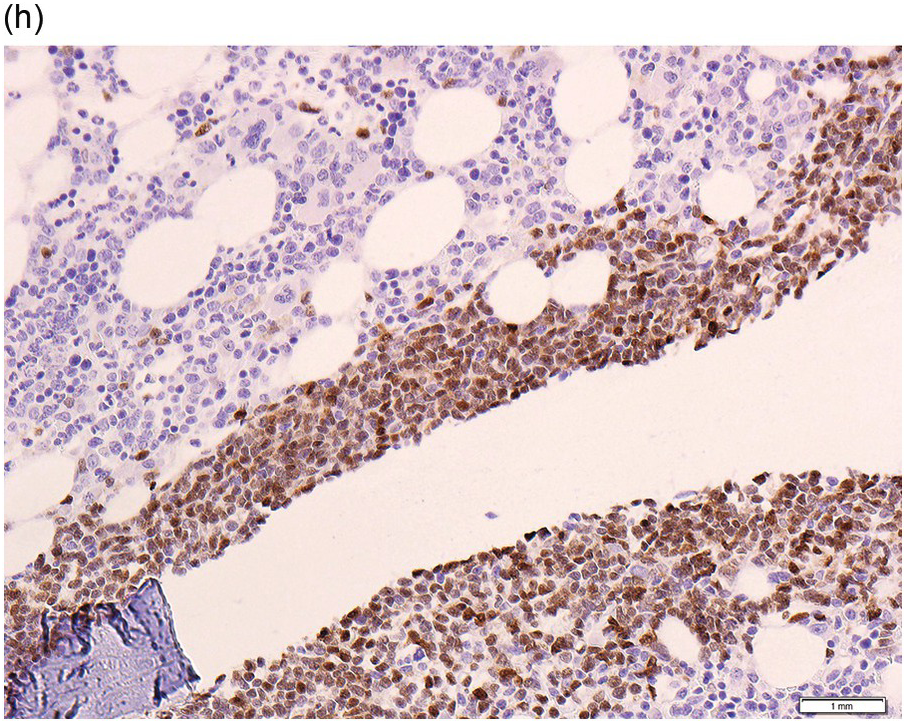
The BMB shows a mixture of interstitial, intrasinusoidal and nodular patterns of involvement (Figure 15.4b, c). The nodular pattern may be more pronounced following splenectomy [26]. The intrasinusoidal component, a useful (but not specific) feature, is better appreciated by CD20 immunostaining, which highlights the characteristic linear configuration of B-cells within sinusoids (Figure 15.4c). The nodules are mostly non-paratrabecular, but may be paratrabecular, and are composed of neoplastic lymphoid cells. They may have underlying follicular dendritic meshworks. Infrequently, the neoplastic cells may surround reactive follicle centres [27, 28]. The neoplastic cells consist of small lymphocytes with round or irregular nuclei. Plasma cell differentiation may be observed and usually forms a minor component but, in some cases, may cause diagnostic confusion with lymphoplasmacytic lymphoma (LPL).
Immunohistochemistry
The neoplastic cells express pan B-markers CD20 and CD79a and are IgM and usually IgD positive. They are negative for CD10, cyclin D1, CD23, LEF1, CD43 and annexin A1 [1]. CD72 (DBA.44) is expressed in a minority of cases [29]. They are usually negative for CD5 but a small subset (which is associated with a high lymphocytosis and a higher proportion showing heavy BM involvement) may be CD5 positive [30]. The lack of cyclin D1 expression may be useful to exclude MCL but absence of SOX11 expression (positive in typical MCL) may not be useful in the leukaemic and splenomegalic setting, when MCL is often SOX11 negative [31].
The immunoprofile of SMZL is not specific but contributes towards excluding lymphoid neoplasms that may have similar cytological features or pattern of BM involvement, which may cause diagnostic confusion.
Genetics
NOTCH1/2 and KLF2 represent the commonest recurrently mutated genes in SMZL [32]. NOTCH2 mutations (up to 40% of cases) are relatively specific for SMZL among B-cell neoplasms and confer a worse prognosis [33]. MYD88 mutation, a genetic alteration that is commonly associated with LPL, is rare in splenic and other MZL and may be a useful discriminator in cases that show plasmacytic differentiation [34].
Hairy Cell Leukaemia
Epidemiology and Clinical Features
Hairy cell leukaemia (HCL) is another rare mature B-cell neoplasm comprising 2% of lymphoid leukaemias and is four to five times more common in men compared to women. Most patients are diagnosed in their middle-age. Patients often present with fatigue, cytopaenias, recurrent infections and hepatosplenomegaly. Monocytopaenia is a hallmark of this condition (Table 15.3).
Blood and Bone Marrow Aspirate
Hairy cell leukaemia has a distinctive appearance in the peripheral blood though tumour cells can be relatively rare. Small- to medium-sized lymphoid cells with oval or bean-shaped nuclei and circumferential, ‘hairy’ cytoplasmic projections are seen (Figure 15.5a). Occasionally larger hairy cells are seen in patients in association with abdominal lymphadenopathy and this is thought to represent a form of transformation. Bone marrow aspiration can be difficult and samples are often haemodilute and aparticulate as mentioned below. When an adequate sample is obtained, mast cells are often prominent in the particles and hairy cells have the same appearance as in the peripheral blood. Hairy cell leukaemia cells have a characteristic immunophenotype (Table 15.3).
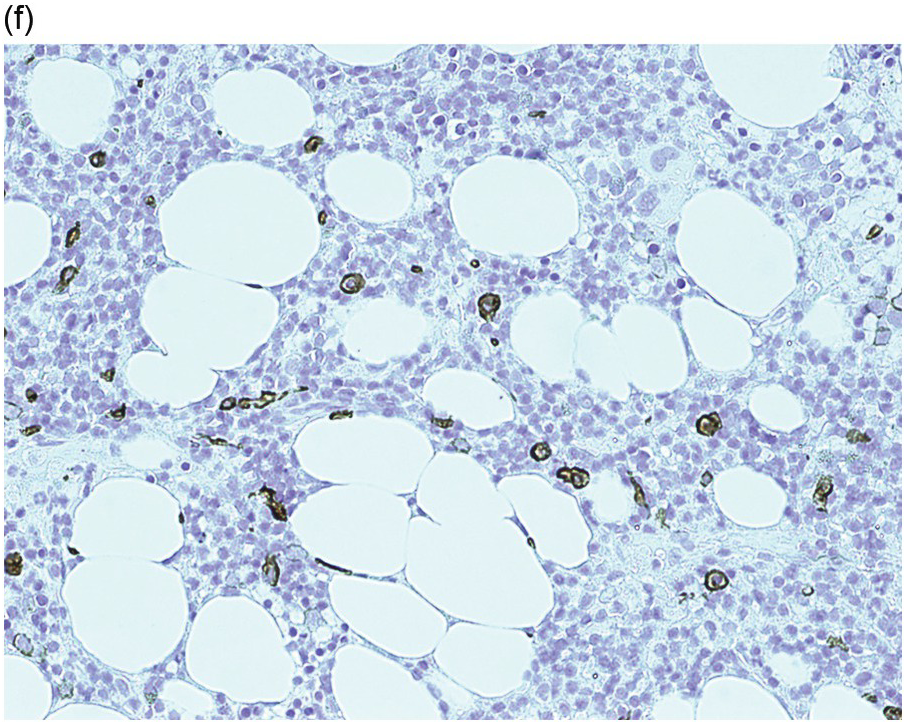
(a) Peripheral blood film shows small- to medium-sized lymphocytes with oval or bean-shaped nuclei and circumferential, ‘hairy’ cytoplasmic projections. (b–d) Bone marrow trephine biopsy. H&E stained sections shows the clustered interstitial infiltrate of neoplastic cells displaying a ‘fried egg’ appearance.
(b) An increase in reticulin is noted underlying the infiltrate.
(c) The infiltrate is positive for cyclin D1.
Bone Marrow Biopsy
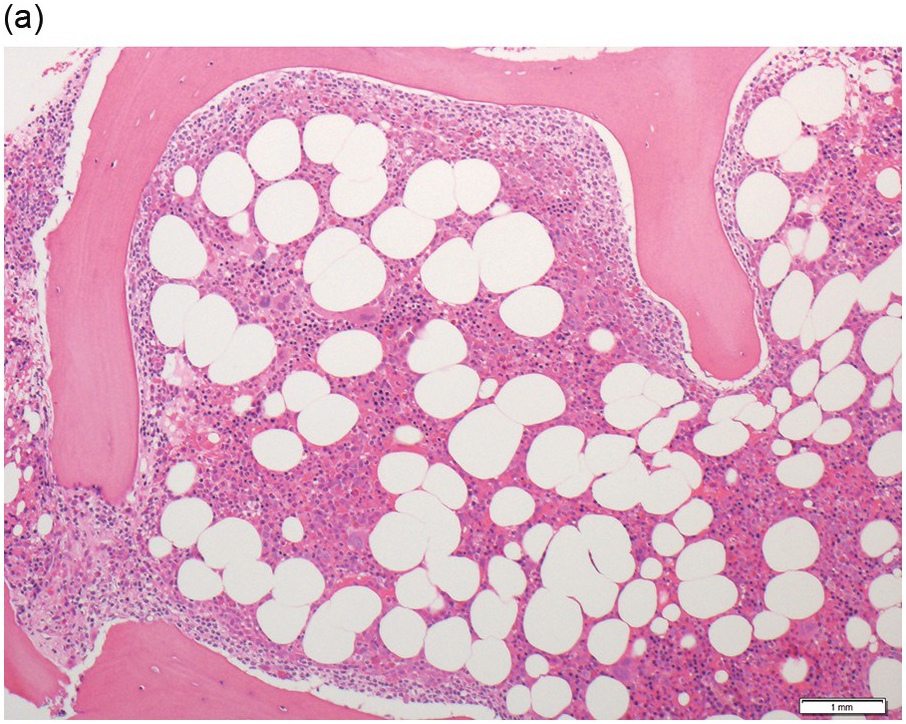
Due to the fibrosis associated with the neoplastic infiltrate, BM aspirates often yield ‘dry taps’. Even in the event a particulate aspirate sample is obtained, the fibrotic foci tend to resist aspiration and therefore the yield may not be representative. BMB is therefore routinely performed at the outset to help confirm the diagnosis and to assess the degree of infiltration prior to treatment and, following therapy, to evaluate the quality of response.
At diagnosis, depending on the extent of infiltration, the involvement is interstitial (most often) or diffuse. If minimal, the infiltrate may be subtle and overlooked. Discrete lymphoid aggregates or marked sinusoidal infiltration, characteristic of other lymphoid neoplasms that may show overlapping clinical presentations, peripheral blood/BM aspirate cytology and immunophenotypic features, are not seen in HCL [5].
The neoplastic cells have abundant clear cytoplasm, well-defined cell margins and widely spaced oval/indented nuclei, which when present in clusters or sheets impart a ‘fried egg’ appearance (Figure 15.5b). There is an increase in reticulin, which is the cause of failed aspirates, and is localized to areas of infiltration and therefore may be diffuse or patchy, depending on the degree of neoplastic infiltration (Figure 15.5c).
It is useful to be aware of the rare multilobulated variant where the nuclei show an unusual multilobulated morphology [35], which may be mistaken for high-grade transformation or another subtype of lymphoma.
Immunohistochemistry
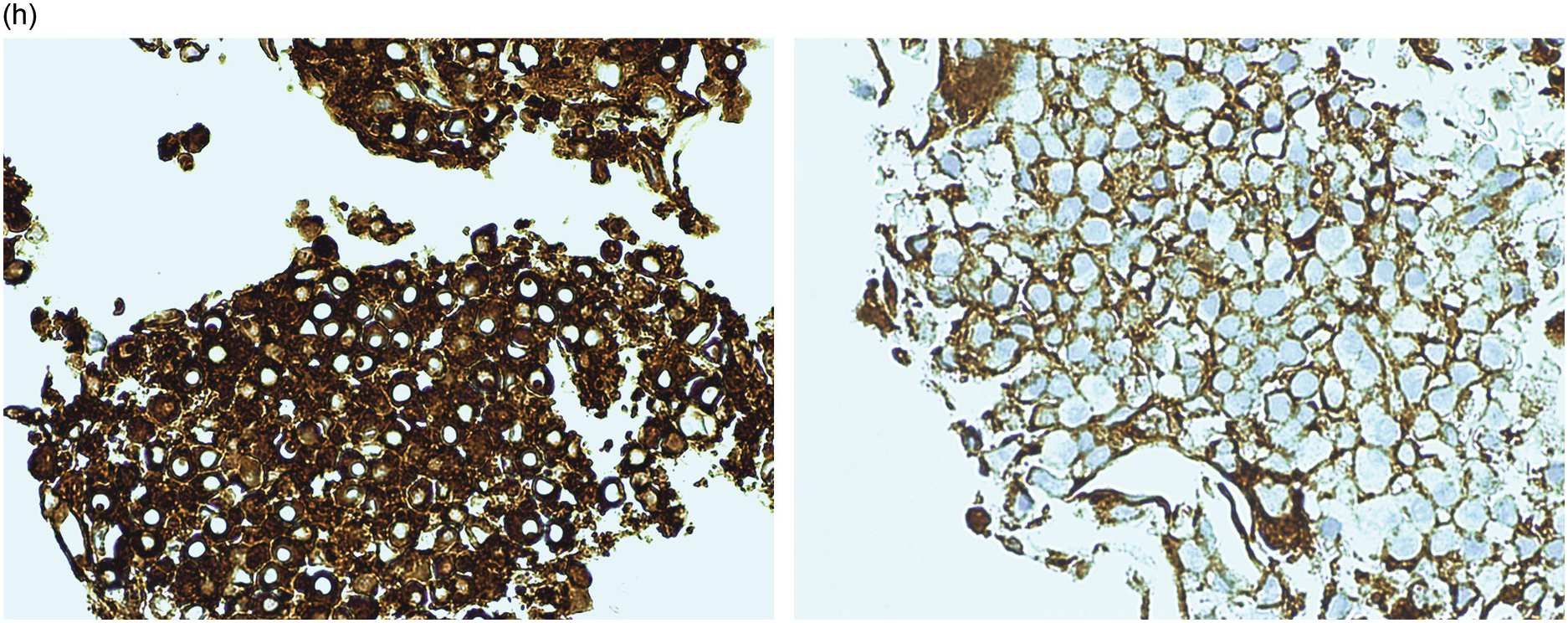
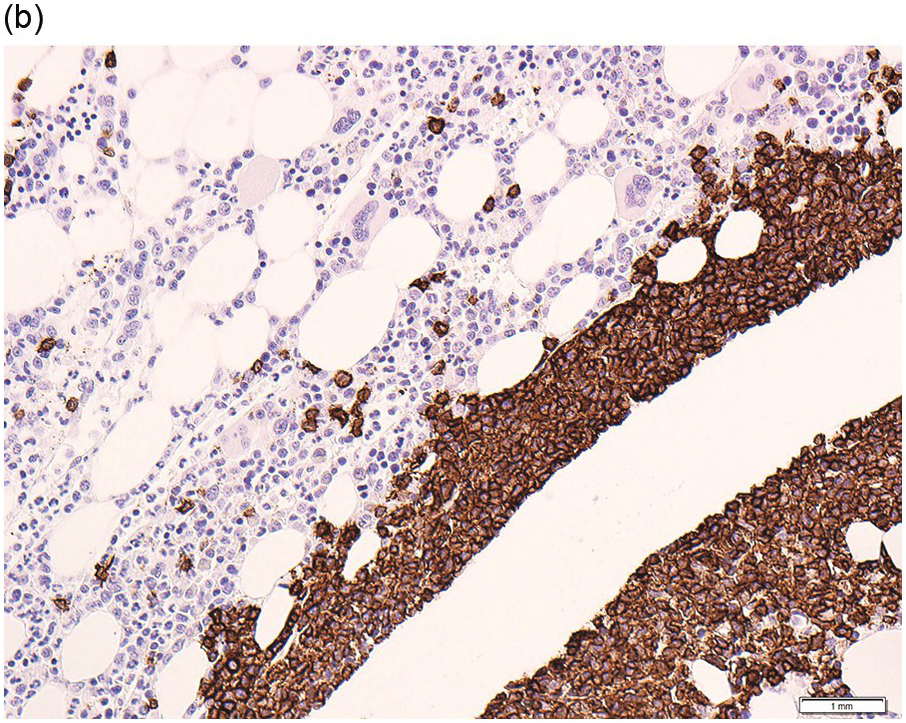
In the event of a failed BM aspirate (‘dry tap’) or paucity of circulating cells when flow cytometry has been unsuccessful, IHC performed on the BMB plays an important role in diagnosis. However, its main role is to quantify the infiltrate at the diagnostic baseline and following therapy.
HCL expresses pan B-markers CD20, CD79a, CD19 and PAX5. It also expresses annexin A1, CD72 (DBA.44), CD11c, CD25, CD123, CD103, TRAP, cyclin D1 (Figure 15.5d) and TXB21 (TBET) [36–38]. Of these markers, annexin A1 is the most specific as it is not usually expressed by other B-cell neoplasms. However, annexin A1 is expressed in myeloid cells and some T-cells and CD11c is expressed by myeloid and monocyte/macrophage cells and therefore their use is often limited to diagnostic BMBs when the HCL infiltrate is heavy and the myeloid population is sufficiently reduced to enable clear distinction between the two populations. Approximately 10% of cases may express CD10 and up to 2% may express CD5 [39, 40].
The BRAF V600E antibody, which identifies mutant BRAF protein by IHC, is a useful marker [41].
Following treatment, the infiltrate may be very subtle requiring pan B-markers CD20 and PAX5 together with TXB21 or cyclin D1, a sensitive (albeit non-specific) marker of HCL, to localize the neoplastic cells. Pan B-markers such as CD19 and CD79a also highlight plasma cells and therefore may not be that useful. Some of the other HCL markers may be useful when used as part of a panel but, as mentioned above, annexin A1 and CD11c are not useful in this setting due to the co-expression of these markers in myeloid cells and/or macrophages. The BRAF V600E antibody may be useful to detect residual disease. Expression of CD25 and cyclinD1 may be downregulated following therapy with the BRAF inhibitor vemurafenib and therefore may not be reliable in the assessment of residual disease in this setting [42].
Splenic B-Cell Lymphoma/Leukaemia Unclassifiable
The WHO classification recognises splenic diffuse red pulp small B-cell lymphoma and HCL variant as provisional entities until they are better characterized and their relationship elucidated, and recommends that those splenic small B-cell lymphomas that do not fulfil the criteria for either of these or SMZL should be designated splenic B-cell lymphoma/leukaemia unclassifiable (Table 15.3) [1].
Splenic Diffuse Red Pulp Small B-Cell Lymphoma
This rare lymphoma shows diffuse red pulp infiltration of the spleen and all cases show a sinusoidal pattern of BM infiltration. Occasionally the BM infiltrate may be exclusively sinusoidal while in other cases it is predominantly intrasinusoidal and accompanied by interstitial or intertrabecular nodular infiltration [6]. Although definite diagnosis may require examination of the spleen, it may be suggested in cases showing exclusive intrasinusoidal infiltration of the BM and villous lymphocytes in peripheral blood and BM aspirate (Figure 15.6a–c) [1].The lymphoma cells typically express CD72 (DBA.44). Please see Table 15.3 for clinical features and peripheral blood, BM aspirate and BMB findings.
Figure 15.6 Splenic diffuse red pulp small B-cell lymphoma (SDRPL) and hairy cell leukaemia variant (HCLv). (a–c) SDRPL. (a) Bone marrow aspirate shows villous lymphocytes with basophilic cytoplasm, similar to splenic marginal zone lymphoma (image courtesy of Dr Megan Nakashima). (b, c) Bone marrow trephine biopsy. H&E stained section showing a predominantly intrasinudoidal infiltrate (b), which is better appreciated on CD20 stain (c). (d) Peripheral blood film of HCLv showing prolymphocytes with hairy projections.
Hairy Cell Leukaemia Variant
Hairy cell leukaemia variant (HCLv) is rare and, although it shows some overlapping clinicopathological features with classic HCL, it shows differing cytological and haematological features, has wild-type BRAF and is resistant to conventional therapy for HCL. It is therefore regarded as biologically distinct from HCL [1]. HCLv presents with clinical manifestations related to the splenomegaly and is associated with a high white cell count but monocytes are within the normal range. The bone marrow is usually aspirable. Cytologically the neoplastic cells have blastic or convoluted nuclei with characteristic prominent central nucleoli (Figure 15.6d). They are readily apparent and easily characterized on peripheral blood, while the BM infiltration may be subtle, and IHC may be required to highlight the infiltrate. Please see Table 15.3 for clinical features and peripheral blood, BM aspirate and BMB findings.
Lymphoplasmacytic Lymphoma
Epidemiology and Clinical Features
Lymphoplasmacytic lymphoma (LPL) has a relatively low incidence among lymphoid neoplasms. It occurs at a median age of 60 years and with a slight male predominance. Waldenström macroglobulinaemia is a subset of LPL characterized by BM involvement and an IgM paraprotein. While lymphadenopathy and constitutional symptoms can be a presenting feature, fatigue and anaemia are probably the commonest presenting features. Other important clinical features include hyperviscosity or neuropathy related to the paraprotein.
Blood and Bone Marrow Aspirate
Circulating lymphoma cells can be seen in LPL as a combination of small mature lymphocytes and lymphoplasmacytic forms that have an oval shape with an eccentric rim of cytoplasm (Figure 15.7a). The characteristic excess of lymphocytes with lymphoplasmacytic forms and plasma cells is seen on a BM aspirate. Flow cytometric immunophenotyping typically detects a clonal population of lymphocytes and plasma cells with the same light chain restriction. Mast cells are often prominent.
Figure 15.7 Lymphoplasmacytic lymphoma. (a) Peripheral blood film showing small mature lymphocytes, lymphoplasmacytoid cells and occasional plasma cells. (b–f) Pre-treatment bone marrow trephine biopsy. H&E stain shows the interstitial lymphoplasmacytic infiltrate (b), which is positive for CD79a (c). The small B-cells and many of the lymphoplasmacytoid cells are positive for CD20 (d) while the plasma cells express CD138 (e). An occasional IgM positive Dutcher body is noted (e, inset). There is an increase in mast cells highlighted by CD117 stain (f). (g, h) Post-treatment trephine biopsy where the neoplastic infiltrate is entirely plasmacytic. The plasma cells (g, H&E stained section) are lambda light chain restricted (h, left panel, lambda; h, right panel, kappa).
Bone Marrow Biopsy
The infiltrate, which consists of small B-cells with varying numbers of plasmacytoid cells and mature plasma cells, is usually nodular and/or interstitial (Figure 15.7) but may be diffuse if heavy. The nodular aggregates may be paratrabecular in location. Dutcher bodies are a frequent finding. The plasma cells, which form clusters amid and around the lymphoid/plasmacytoid infiltrates, display a mature morphology. A characteristic increase in mast cells is often present amid the infiltrate [46].
Despite the features described it is difficult, on morphology alone, to confidently exclude other small B-cell neoplasms that may show plasmacytic differentiation. Even with the use of IHC this may be difficult or impossible in cases of BM involvement by nodal or extranodal MZL [46]. Some cases of SMZL may also cause morphologic and immunophenotypic overlap.
Post treatment the BM may show a reduction in the lymphoid component with a persistence and therefore relative increase in the plasmacytic component (Figure 15.7g, h). In the absence of a previous history, if the residual infiltrate is purely plasmacytic it may cause confusion with plasma cell myeloma (PCM) although, as stated below, the immunoprofile of the plasma cells would help resolve this [47].
Immunohistochemistry
The LPL infiltrate expresses CD79a and CD19 (Figure 15.7c). The small B-cells and varying numbers of plasmacytoid cells express CD20 and PAX5 (Figure 15.7d). They often express IgM (less frequently IgG and rarely IgA), which is best appreciated in the plasmacytoid/plasmacytic cells that may also contain IgM (or IgG/IgA) positive Dutcher bodies (Figure 15.7e inset). The infiltrate is usually negative for CD5 but a small number of CD5 positive examples may be encountered. Although typically CD23 negative, a subset of cases may be variably positive for CD23 [46]. The infiltrate is usually IgD negative. The plasma cells are light chain restricted and are positive for CD138 (Figure 15.7e) and CD19, may show variable PAX5 expression and are negative for CD56 and cyclin D1. This contrasts with neoplastic plasma cells in PCM, which typically show loss of CD19 and may co-express CD56 and cyclin D1 [48]. This differing immunoprofile is especially useful in distinguishing post-treatment cases where the plasmacytic component alone persists (Figure 15.7g, h) from involvement by PCM, and also to distinguish between the coincidental occurrence of a plasma cell dyscrasia/monoclonal gammopathy of undetermined significance (MGUS) and an unrelated small B-cell neoplasm from plasma cell differentiation in LPL. When present, cyclin D1 is typically strongly positive and therefore a very useful discriminator in the small subset of PCM that has a small, rather uniform, lymphoplasmacytoid morphology, often expressing CD20 and which may be positive for PAX5 [49].
CD117 may be useful to highlight the increase in mast cells (Figure 15.7f).
Genetics
Until recently there has been no specific immunophenotypic or genetic marker that enabled distinction from other small B-cell neoplasms with plasmacytoid/plasmacytic differentiation. Recurrent point mutations in the MYD88 gene, where proline replaces leucine at position 265 (L265P) are seen in 67 to 100% of cases; it is rare in other small B-cell neoplasms and has hitherto not been reported in plasma cell myeloma [50–52]. Mutations in CXCR4 are found in approximately 30% of WM cases and are unique to this neoplasm [53]. They are always found in association with MYD88 mutations and, when present, are strongly associated with heavier BM infiltrates as well as higher paraprotein levels.
Nodal Marginal Zone Lymphoma and Extranodal Marginal Zone Lymphoma of Mucosa Associated Lymphoid Tissue
BM involvement in nodal marginal zone lymphoma (NMZL) and extranodal marginal zone lymphoma of mucosa associated lymphoid tissue (MALT lymphoma) may show similar morphological or immunophenotypic features that may also overlap with those of LPL and SMZL. However, BM involvement by either NMZL or MALT lymphoma may be confirmed when BMB is performed as part of staging investigations. The pattern of BM involvement may be focal, paratrabecular and non-paratrabecular and/or interstitial [8].
Follicular Lymphoma
Epidemiology and Clinical Features
Defined by the WHO as a neoplasm composed of follicle centre B-cells (typically both centrocytes and centroblasts), which usually has at least a partly follicular pattern, follicular lymphoma (FL) is the commonest indolent lymphoma constituting 25 to 30% of all lymphomas. Those lymphomas composed of centrocytes and centroblasts with an entirely diffuse pattern in the sampled tissue may also be included in this category [1].
Most patients have disseminated disease with lymphadenopathy with or without splenomegaly. Bone marrow involvement may be seen in 40 to 70% of cases [1]. Although BM involvement may determine stage IV disease, it often does not impact on therapy-related decisions in cases with disseminated extramedullary disease. However, if the extramedullary presentation is localized, a positive BMB would alter the stage from I to IV and may change management strategy.
Bone Marrow Aspirate
The degree of lymphoid infiltration, seen as small- to medium-sized mature lymphocytes with cleaved nuclei, can vary widely on the aspirate. In some instances, despite lack of obvious lymphocyte excess, flow cytometry can detect low level infiltration. Typically, FL cells exhibit a mature B-cell phenotype along with expression of surface CD10 in the vast majority of cases on immunophenotyping.
Bone Marrow Biopsy
BMB is usually performed as a staging investigation with a specific diagnosis of lymphoma already established on extramedullary biopsy, often a lymph node. The BM involvement by FL may be concordant with FL or discordant, with DLBCL diagnosed on the extramedullary biopsy [2].
BM involvement shows characteristic paratrabecular localization (Figure 15.8a, b), a pattern that is not usually seen in benign lymphoid aggregates. The infiltrate may spill over in an interstitial pattern into surrounding BM. Infrequently a follicular pattern with underlying FDC meshworks may be seen, when the nodular involvement may be non-paratrabecular or show a mixture of paratrabecular and non-paratrabecular patterns [54].
Figure 15.8 Follicular lymphoma and mantle cell lymphoma. (a, b) Follicular lymphoma. Bone marrow trephine biopsy showing the characteristic paratrabecular infiltrate of small irregular lymphoid cells (a, H&E; b, CD20 stain). (c–h) Mantle cell lymphoma. (c) Peripheral blood film showing small mature lymphocytes displaying nuclear clefts with a ‘fish-mouth’ appearance. (d–f) Bone marrow trephine biopsy of mantle cell lymphoma showing striking paratrabecular infiltration. (d, e). H&E stained section showing paratrabecular infiltration by small lymphoid cells with irregular indented nuclei. The infiltrate is positive for CD20 (f), CD5 (g) and cyclinD1 (h).
The neoplastic cells in the BM often resemble the interfollicular component in the lymph nodes and mainly consist of centrocytes. In some cases, there may be a mixture of centrocytes and centroblasts with the former predominating.
Immunohistochemistry
The expression of BCL2 is not of diagnostic value in excluding other lymphomas. Similar to the interfollicular component in lymph nodes, FL often shows downregulation of germinal centre markers in BM infiltrates. However, Younes et al. showed that although BCL6 and LMO2 were not of diagnostic use in this context, CD10 and HGAL expression was seen in 47 and 58.8% of cases, respectively. Expression of CD10 and HGAL correlated well with the presence of underlying FDC meshworks [54].
As BM evaluation is usually a staging investigation following an established diagnosis, detailed immunophenotypic characterization of the BM infiltrates may not be required and the identification of paratrabecular B-cell aggregates, a pattern typical of FL, is sufficient to confirm BM involvement.
Genetics
The genetic hallmark in FL is the presence of the IGH/BCL2 translocation. More recently, next-generation sequencing studies have revealed the importance of epigenetic dysregulation in FL. MLL2 mutations are the most frequent and are present in nearly 90% of cases. Other recurrent mutations seen at a lower frequency include CREBBP and EZH2 mutations, which are present in up to a third of patients [55].
Mantle Cell Lymphoma
Another rare but interesting entity among B-cell neoplasms, mantle cell lymphoma (MCL) can show extreme heterogeneity in clinical presentation, ranging from indolent to aggressive. The vast majority of patients present with advanced-stage disease and intestinal involvement is common. While characteristics such as isolated leukaemic disease (leukaemic, non-nodal MCL), low proliferation index, hypermutated IGH and kappa light chain restriction have been associated with indolent disease [31, 56, 57], there is no single marker at present, including lack of SOX11 expression [58] that can reliably predict an indolent course.
Blood and Bone Marrow Aspirate
Circulating lymphoma cells are detectable in the majority of patients but approximately 20 to 30% of patients present with high white blood cell counts. Circulating lymphoma cells are small- to medium-sized and display mature nuclei that often have a cleft with a ‘fish-mouth’ appearance (Figure 15.8c). Patients with pleomorphic and blastoid variants can display larger atypical lymphoid cells in circulation and occasionally typical prolymphocytic morphology with a single prominent nucleolus. The BM aspirate can show variable infiltration. Immunophenotyping of blood and BM can be very informative. The majority of MCL express CD5 and important differences in surface markers compared to CLL include the lack of CD23 and CD200.
Bone Marrow Biopsy
BMB, which is often involved in MCL, is usually a staging investigation when diagnosis has previously been established on biopsy of an extramedullary site. However, less commonly, especially in cases with a leukaemic/splenomegalic presentation, BM may represent the site of initial biopsy. The pattern of distribution may be nodular non-paratrabecular, paratrabecular, interstitial or a mixture of patterns. If the infiltrate is heavy, it may also be diffuse [2].
The infiltrate in the majority of cases is monomorphic and composed of small- to medium-sized cells with irregular/indented nuclei, resembling centrocytes. Chromatin is dispersed with inconspicuous nucleoli. The less frequent morphological variants are listed in Table 15.4 [1]. The aggressive blastoid and pleomorphic variants may be seen at presentation or at progression/relapse.
Table15.4 Morphological variants of mantle cell lymphoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree