Clinical Relevance
Kidney failure is a growing problem in the older population. Data on people reaching end-stage kidney disease (ESKD) is collected by the U.S. Renal Data System (USRDS). All dialysis units that receive funding from Medicare are required to file data with the USRDS, so that nationwide data are available on over 95% of people receiving renal replacement therapy. Information published in the 2006 USRDS annual data report shows that approximately 1.5 in 1000 persons aged 65 years or older are initiating treatment for ESKD each year—the highest rate of any age group. Over the last 10 years, the number of older people enrolling for treatment has increased by 41% in the group of people aged 75 years or older and by 48% in the 80+-year-old age group. Almost 4 in 1000 persons are currently maintained on renal replacement therapy, with the 75+-year-old age group growing at 10% per year. The peak incidence for ESKD is the 70- to 74-year-old age group, while the peak prevalence falls in the 65- to 69-year-old age group. In contrast, the incidence of ESKD in the 20- to 44-year-old age group has remained flat over the last 10 years, with only 6% growth in the 45- to 64-year-old group. Although some of the increase in renal replacement therapy for the older population indicates a greater willingness to offer treatment to older individuals, much of the increase is owing to people surviving to experience the chronic changes that occur with aging. The kidney undergoes significant age-related change. Other common diseases such as hypertension and diabetes accelerate these changes.
The Aging Process
Aging in the kidney is characterized by changes of both structure and function. It must be emphasized that many of the aging studies have been performed on laboratory animals, particularly rodents, that demonstrate quite different patterns of aging from humans. For example, kidney weight increases throughout life in rats while kidney mass and size in humans peaks in the fourth decade and declines thereafter. Care should be taken when reading the literature to keep in mind that changes seen in animal models may not be reflected by parallel changes in humans. Historical data from human postmortems describing changes in the kidney made no effort to exclude patients with kidney disease or significant comorbidities. More recently data on aging has been developed from longitudinal studies, such as the Baltimore Longitudinal Aging Study, in which the medical histories of the study volunteers are well documented. There are also data accumulating from the kidney transplant population. Older living donors are increasingly being used and are put thorough a rigorous medical workup for renal function and comorbid conditions before being accepted as donors. This has allowed acquisition of data on normal aging in the kidney, uncomplicated by the presence of medical comorbidities. Aging in the kidney is generally characterized by spontaneous progressive decline in renal function accompanied by thickening of the basement membrane, mesangial expansion, and focal glomerulosclerosis.
Changes in renal function with age are well documented both in human and animal models. Although baseline homeostasis of fluids and electrolytes is maintained with normal aging, there is a progressive decline in renal reserve. This results in a compromise in the kidney’s ability to respond to either a salt or water load or deficit. This manifests clinically in patients being vulnerable to superimposed renal complications during acute illnesses. Chronic conditions such as hypertension accelerate this age-related loss of renal reserve and increased vulnerability in these patients should be anticipated. Age-related changes in function will be considered by separate functional domain within the kidney.
Average renal blood flow decreases about 10% per decade, dropping from 600 mL per minute per 1.73 m2 to 300 mL per minute per 1.73 m2 by the ninth decade. This is accompanied by increasing resistance in both afferent and efferent arterioles. These changes occur independent of cardiac output or reductions in renal mass. This decline in renal blood flow is thought to contribute to the decline in efficiency with which the aging kidney responds to fluid and electrolyte load and loss.
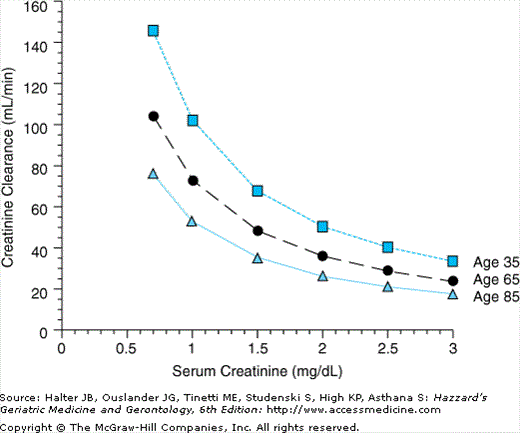
Newer data have shown a wide variation in the rate and extent of changes in the kidney within the older population. Approximately 30% of the population shows no measurable decline in renal function with normal aging. The bulk of the population loses about 10% of glomerular filtration rate (GFR) and 10% of renal plasma flow per decade after the fourth decade of life. Between 5% and 10% of the population shows accelerated loss, even in the absence of identifiable comorbidities. Since there is also a steady loss of muscle bulk with age, with concomitant reduction in creatinine production, serum creatinine should remain constant. Rises in serum creatinine should therefore be taken seriously and not dismissed as normal aging. As can be seen in Figure 85-1, serum creatinines at the upper limit of the “normal range” in an older individual represent significant functional decline, and thought should be given to renal dosing of medications. These curves were calculated using the Cockcroft Gault equation for a 70-kg man:
Results for women should be multiplied by 0.85, which shifts the curves downwards. In frail older women with very little residual muscle mass, this equation probably overestimates GFRs. This steady decline in renal function with age manifests itself clinically as impaired ability to excrete a salt or water load. Extra care should be taken when replacing fluids in an older individual to prevent extracellular fluid overload.
In 1999, an improved formula for estimating GFR was developed known as the MDRD formula (because it was developed as part of the Modification of Diet in Renal Disease study). Many routine laboratories now automatically calculate an MDRD glomerular filtration estimate when a basic or a comprehensive metabolic panel is ordered. In some institutions, it is necessary to order a “renal panel” for the calculation to be done. It is important to understand that this formula was based on data from community-dwelling volunteers aged 18 to 70 years. It has never been validated in an elderly or frail population. Several investigators have studied its performance in elderly patients and compared its efficacy with Cockroft Gault, creatinine clearances based on 24-hour urines or iothalamate clearances, or a combination of these methods. All of the studies have shown large discrepancies between these methods in patients with advanced age and at both extremes of the weight spectrum. These limitations should be kept in mind when using the formula in clinical geriatric practice. Although iothalamate clearance is the gold standard, it is expensive and impractical for routine use. The most reliable results come from calculating creatinine clearances based on 24-hour urine collection. This will always be an overestimate of the GFR, since some creatinine is actively excreted into the urine from the proximal tubule, not all of the urinary creatinine is filtered. It is however more reliable than the formula estimations in the very old and the frail.
The classification of kidney disorders has undergone a major revision over the past few years. A consensus committee, sponsored by the National Kidney Foundation, published new clinical practice guidelines in February 2002. The traditional chronic renal insufficiency (CRI) has become chronic kidney disease (CKD) and end-stage renal disease (ESRD) has become kidney failure. CKD is defined as either kidney damage or decreased kidney function for 3 or more months. Kidney failure is defined as a GFR of less than 15 mL/min or the need to start kidney replacement therapy. Along with renaming kidney disease, the committee also developed a system of staging, similar to the New York classification of congestive heart failure. It was felt that having a structure would help with standardizing diagnosis and opportunities for preventative management. CKD is now classified into five stages, regardless of underlying diagnosis. The classification defines stage 1 as kidney damage (primarily proteinuria) with preserved GFR and progresses to stage 5 kidney failure (Table 85-1). Declines in GFR are accompanied by a broad range of complications (Table 85-2). Early recognition of impaired kidney function allows the physician to screen for and manage these complications and thus prevent comorbidities and declines in quality of life. National Kidney Foundation Guidelines recommend referral to a nephrologist when a patient reaches stage 4 CKD for management of the complications of impaired function such as acidosis, phosphorus retention, and anemia. Preparation for kidney replacement therapy should also begin during stage 4.
STAGE | DESCRIPTION | GFR (mL/min/1.73 m2) |
|---|---|---|
1 | Kidney damage with normal or increased GFR | >90 |
2 | Kidney damage with mild reduction in GFR | 60–89 |
3 | Moderate decrease in GFR | 30–59 |
4 | Severe decrease in GFR | 15–29 |
5 | Kidney Failure | <15 (or dialysis) |
Hypertension |
Anemia |
Increased cardiovascular mortality and morbidity |
Disorders of calcium and phosphorus metabolism |
Compromised nutritional status |
Metabolic bone disease |
Neuropathy |
Impaired functioning and well-being |
Depression |
Despite the significant decline in GFR that occurs with aging, proteinuria is not a normal feature of the aging process. Proteinuria is always a pathological finding and requires a full workup. In contrast, in most rodent models, particularly in the rat, proteinuria is a normal feature of the aging kidney. This difference between humans and rodents should be kept in mind when reading the aging literature.
Older individuals are well known to be more susceptible to acute renal failure. Much of the information on tubular function comes from animal studies, particularly rat models. Rats spontaneously develop proteinuria with aging, and this protein load is believed to be toxic to the tubule. Since proteinuria is not a feature of normal aging in humans, these animal studies may not paint an accurate picture of changes in tubular function in humans. There are also large numbers of studies in experimental animals looking at vasoconstrictive and vasodilatory responses in the older kidney. Impaired response to ANP, acetylcholine, and blunted responses of cAMP to β-adrenergic stimulus have all been implicated. Virtually none of these findings have been confirmed in humans. Functional magnetic resonance imaging (MRI) in older volunteers has demonstrated decreased ability to modulate renal medullary oxygenation. Whether this is caused by fixed vascular changes or changes in renal autocrine systems such as prostaglandins, dopamine, nitric oxide (NO), naturetic peptides, or endothelin is not clear. The clinical result is increased sensitivity to acute ischemic renal failure.
Animal and human studies have shown impaired concentrating ability in the older kidney. Whether this is caused by intrinsic defects in the tubular epithelium or impaired response to ADH is not clear. Studies have also demonstrated impaired capacity to acidify urine manifested clinically as reduced excretion of an acid load. Whatever the underlying mechanism, older individuals are less likely to be able to maintain normal homeostasis when challenged. Although there is an age-related decline in tubular functions such as glucose and amino acid transport, these declines closely parallel the decline in GFR and are believed to correlate with the loss of nephrons rather than aging of the tubule. Age-related changes in sodium and potassium homeostasis, and water handling are discussed in Chapter 88.
Older individuals are also more sensitive to nephrotoxic injury. Careful thought should be given to the choice and dosing of antibiotics and other nephrotoxic drugs. Increased age is a risk factor for the development of radiocontrast nephropathy. Special care should be exercised before tests requiring radiocontrast are ordered.
Age also impacts on the viability of kidneys for transplantation. With the steady increase in living related and unrelated donations, more organs have become available for use in the older population. Although nationwide probably less than 5% of older individuals reaching ESKD are being considered for transplantation, many larger transplant programs are routinely offering transplantation to people in their late sixties and early seventies if they are otherwise in good health. Donated organs are commonly coming from a similar aged spouse or a family member. Age of the donated organ appears to be an independent risk factor for graft survival. This does not appear to be immune-mediated, as older recipients have lower risk of rejection. These organs typically show delayed graft function posttransplant, with chronic allograft nephropathy and result in higher baseline serum creatinines in the long term. It has been postulated that this is caused by impaired response to injury in the older kidney, but there is no real scientific evidence to support this at the current time.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree