INTRODUCTION
Invasive cervical cancer is relatively rare in developed countries, and only about 12,000 new cases are diagnosed each year in the United States.
1 However, invasive cervical cancer remains a major public health problem in medically underserved communities and is the most common cause of cancer death for women in many developing countries. The high frequency of cervical cancer in young mothers contributes to a disproportionate socioeconomic impact of the disease.
As discussed in
Chapter 1, more than 99% of cervical carcinomas arise as a direct result of sexual transmission of human papillomavirus (HPV), which in some cases leads to the development of intraepithelial neoplasia and ultimately invasive cervical cancer. The long preinvasive phase of most cervical cancers has enabled successful prevention programs based on cytologic screening, screening for high-risk HPV, and treatment of high-grade squamous intraepithelial lesions. Primarily because of differences in access to screening and treatment, there are large socioeconomic, racial, and national disparities in the incidence rates of invasive cervical cancer. For example, in the United States, the incidence of invasive cervical cancer in non-Hispanic White women is about half that in Black women and about 70% that in Hispanic women; the incidence in women whose family income is at least twice the poverty threshold is one-third to one-half the incidence in women who have lower family incomes.
2Although safe sexual practices reduce the risk of HPV infection, recent development of an effective HPV vaccine offers the greatest chance for achieving a major reduction in the incidence of cervical cancer (
Chapter 1). However, to be effective, the vaccine must be administered before infection occurs. Because of the long latent period from HPV infection to development of invasive cervical cancer and because of low vaccination rates in the United States and elsewhere, it will be many years before introduction of the HPV vaccine has a major impact on cervical cancer incidence.
Cervical cancer was one of the first cancers to be treated successfully with radiation therapy (RT); the first encouraging reports of cervical cancer treatment with radiation came within 5 years of the discovery of radium. There remain few diseases for which the radiation oncologist plays a more central role in treatment and few diseases that pose more difficult management challenges.
Because the close proximity of the cervix to the bladder, ureters, and rectum limits the resectability of bulky cervical cancers, RT is usually the treatment of choice for locoregionally advanced disease. Definitive RT is highly effective in the treatment of such cancers; however, the best results are achieved with combinations of external beam irradiation, brachytherapy, and chemotherapy that are technically complex and have significant acute adverse effects. This complicated multimodality treatment makes the management of this disease particularly challenging. Cure of locoregionally advanced cervical cancer requires control of local disease. Distant metastases are rarely present at diagnosis, and even cancers that are locoregionally extensive can often be cured if locoregional control can be achieved (CS 10.6 to 10.10). The salvage options for most recurrences of locoregionally advanced disease are poor, and thus, the success of initial treatment is particularly crucial. However, patients who have localized recurrences in unirradiated sites can sometimes be cured with definitive chemoradiation (CS 10.7 and 10.9).
For patients treated with initial hysterectomy, adjuvant RT reduces pelvic recurrences and probably improves survival (CS 10.1 and 10.2).
The demographics of cervical cancer can limit access to high-quality care in two major ways. First, most cases occur in regions of the world that are medically underserved and have limited access to the diagnostic tools and treatments that may yield the best results. Second, in developed countries, the rarity of cervical cancer cases makes it difficult for most clinicians to maintain the specialized technical skills required for optimal disease management. In particular, in the United States, where RT has become increasingly decentralized, most nonacademic practices treat fewer than two to three cases of intact cervical cancer per year. Surveys have revealed that patients treated in nonacademic practices are, on average, more likely to have protracted or incomplete treatment and less likely to receive brachytherapy than those treated in academic centers.
3 These disparities, the reasons for which are probably multifactorial, highlight some of the challenges faced by radiation oncologists and their cervical cancer patients.
This chapter focuses on the factors used to guide treatment choices for cervical cancer and the strategies used to combine multiple modalities to deliver treatment optimally. Technical details of external beam irradiation and brachytherapy are discussed in greater detail in
Chapters 6 and
8, respectively.
PRETREATMENT EVALUATION AND STAGING
Thorough and accurate pretreatment evaluation is a critical step in the curative treatment of cervical cancer. The goals of pretreatment evaluation are as follows:
To determine the extent of the primary tumor. Although even large cervical tumors may remain grossly confined to the cervix, tumors also may infiltrate the adjacent supporting ligaments (
Chapter 5), vagina, uterine body, and, rarely, bladder or rectum. The size and distribution of cancer in the cervix and uterus and the presence and pattern of paracervical tumor infiltration influence the design of external beam RT fields and the details of subsequent brachytherapy.
To determine whether hydronephrosis is present. Locally advanced tumors can cause ureteral compromise that influences the stage of disease and, if untreated, can affect chemotherapy tolerance (
Chapter 7).
To determine the extent of regional disease. Cervical cancers generally follow a fairly orderly pattern of regional spread to pelvic and, secondarily, paraaortic nodes (
Chapter 5). Even extensive nodal disease can often be cured if it is detected and treated with an adequate radiation dose.
To determine whether distant metastases are present. Although extremely rare in patients with early-stage squamous carcinomas of the cervix, distant metastases are sometimes present at diagnosis in patients who have very extensive local disease or high-risk histologic types, such as small cell neuroendocrine cancer. For patients who have distant metastases, chemotherapy is rarely, if ever, curative; distant metastases should always be confirmed histologically before a decision is made not to administer potentially curative local treatment.
To determine the patient’s overall medical condition. All patients require a detailed history and physical examination to evaluate their level of pain and distress and to identify any medical conditions that could affect their ability to tolerate treatment.
To assign an FIGO (International Federation of Gynecology and Obstetrics) stage. All patients should have a stage assigned, using the FIGO clinical staging system (
Table 10.1).
All patients with cervical cancer require standard laboratory studies, including complete blood cell count, measurement of electrolyte levels, and renal and liver function studies, to determine their fitness for treatment.
The following studies are particularly useful in the evaluation of patients with cervical cancer:
Physical examination. Even advanced imaging techniques do not obviate the need for a careful physical examination. The size, morphology, and mobility of the tumor and the presence of paracervical and vaginal extension should be carefully noted and described. If there is more than minimal vaginal involvement, fiducial markers can be placed at the distal extent of disease to help with treatment planning for external beam irradiation and subsequent brachytherapy. Pelvic examination is discussed in more detail in
Chapter 4.
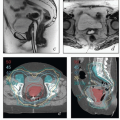
Magnetic resonance imaging (MRI). The excellent soft tissue detail provided by MRI makes it the most useful study for the evaluation of the cervix and paracervical tissues.
6,7 However, it should be remembered that there are few published studies on the accuracy of MRI findings (particularly those regarding early paracervical involvement) in patients with locally advanced cervical cancer (
Chapter 4). For patients with early-stage disease, MRI can be used to estimate the extent of cervical stromal involvement and may be helpful in determining whether patients should be treated with primary surgery or primary RT. For patients with adenocarcinoma involving the cervix, MRI can be used to evaluate the distribution of disease in the cervix and uterus and suggest the most likely primary tumor site. MRI can also yield important information about the extent of paracervical involvement, the position of the uterus, and the conformation of the cervicouterine canal—details that are particularly important for brachytherapy treatment planning. MRI can also provide information about the disease status of regional nodes and clarify the nature of fluorodeoxyglucose (FDG)-avid pelvic wall lesions (e.g., differentiate involved nodes from physiologically active ovaries). As discussed in
Chapter 8, MRI is also increasingly being used for imageguided brachytherapy
(CS 10.4 and 10.5).
Cystoscopy and proctoscopy. Any patient who has findings suggesting bladder or rectal involvement on MRI or physical examination should have cystoscopy to confirm or rule out bladder involvement or proctoscopy to confirm or rule out rectal involvement. Biopsy proof of bladder or rectal mucosal involvement is required to classify cervical cancer as stage IVA (
Table 10.1).
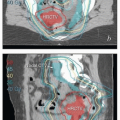
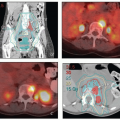
Positron emission tomography-computed tomography (PET-CT). PET-CT is currently the most accurate noninvasive method for detecting nodal metastases from cervical cancer.
8,9 PET-CT can also detect hydronephrosis and is a sensitive method for detecting distant disease. PET-CT is extremely helpful in treatment planning for patients with extensive nodal disease. For these reasons, PET-CT is recommended for most patients with greater than stage IB1 cervical cancer. PET-CT is also useful in the evaluation of response to treatment (
Chapter 7). For further discussion of the advantages and limitations of PET, see
Chapter 4.
Contrast-enhanced CT. If PET is unavailable, contrastenhanced CT of the chest, abdomen, and pelvis can be used for detection of nodal and distant metastases, although the sensitivity of this technique is lower than that of PET-CT. Contrast-enhanced CT can also provide some useful information about the size of cervical tumors, although the soft tissue detail is far inferior to that of MRI.
For patients who are to have surgical treatment for stage IA or IB1 cancers, physical examination, chest radiography, and laboratory studies are usually sufficient for preoperative evaluation; in selected cases, MRI may help to select the best initial treatment by delineating the extent and distribution of tumor in the cervix and uterus. For patients with more advanced lesions, the ideal evaluation probably includes pelvic examination, MRI of the pelvis, and PET-CT, with endoscopic studies reserved for patients who have radiologic evidence of organ involvement. All patients who have neuroendocrine tumors should be evaluated for the presence of metastatic disease; this evaluation usually includes CT or MRI of the brain, although the yield is low for patients who do not have other evidence of distant disease.
In low-resource settings, CT of the chest, abdomen, and pelvis may be performed in place of MRI or MRI and PET-CT as a less sensitive but still useful method of detection of locoregional and distant disease.
Although nodal findings from tomographic imaging studies do not influence stage in the current FIGO clinical staging system, some form of imaging to rule out gross nodal involvement should always be performed because the incidence of nodal involvement in cervical cancer is high and failure to deliver a sufficient dose of radiation to sites of gross disease is likely to lead to fatal infield disease recurrences. Patients with locoregionally advanced disease also require some form of imaging to rule out the presence of hydronephrosis.
RISK ASSESSMENT
Before the clinician can offer informed treatment recommendations or counsel the patient about her prognosis, a thorough analysis of important risk factors for pelvic disease recurrence, nodal and distant metastases, and death from cervical cancer is necessary. Although all newly diagnosed cancers are classified in terms of FIGO stage, treatment decisions and assessment of prognosis are heavily influenced by factors not reflected in or incompletely addressed in the FIGO staging system.
FIGO Stage
The internationally accepted FIGO clinical staging system is based primarily on findings from physical examination, plain film radiography, and, where appropriate, cystoscopy, proctoscopy, and cone biopsy (
Table 10.1). Although FIGO stage is correlated with outcome, it does not take into account many of the most important prognostic indicators, most notable among them the presence of nodal metastases. In the FIGO clinical staging system, most findings that are detectable by modern tomographic imaging studies, including distant metastases, cannot be used to alter the clinical stage unless these findings are also detectable by physical examination or other approved studies.
Since its inception in the early 20th century, the FIGO clinical staging system has had the same four major categories. However, the system has been amended a number of times, most notably to refine the definition of stage IA in 1994 and to subdivide the stage IB and IIA categories according to tumor diameter in 1994 and 2009, respectively. Also in 2009, FIGO for the first time permitted tomographic imaging findings to influence primary tumor classifications, stating, “The use of diagnostic imaging techniques to assess the size of the primary tumor is encouraged but is not mandatory. Other investigations (i.e., examination under anesthesia, cystoscopy, sigmoidoscopy, intravenous pyelography) are optional and no longer mandatory.”
4 Presumably, the “other investigations” can only be excluded if some other method is used to assess for bladder, ureteral, and rectal involvement.
Under the 2009 FIGO clinical staging system, regional metastases detected by CT, MRI, or PET still cannot be used to alter the FIGO stage; as a result, only rare patients who have palpable pelvic lymphadenopathy can have their stage increased (to stage IIIB) on the basis of nodal metastases. This policy has been maintained by FIGO for several reasons:
Most cervical cancers are diagnosed in underdeveloped countries that have limited access to advanced imaging methods.
Most locally advanced cervical cancers are not surgically staged.
Because the methods used to evaluate nodal involvement (CT, MRI, PET, lymphadenectomy, etc.) have widely varying sensitivities and specificities, their incorporation into the staging system would cause hidden inconsistencies that would confound outcome comparisons.
FIGO has defined a supplemental pathologic staging system for patients who undergo primary treatment with radical hysterectomy and lymphadenectomy (
Table 10.2).
This system is meant only for patients who undergo primary surgical treatment; it should never be applied in other cases. Unfortunately, the FIGO pathologic staging system is often published without the rules for its application, causing it to be misapplied in various ways to cases of intact cervical cancer and resulting in considerable confusion. The fact that the same stage labels (I, II, III, and IV) are used for the clinical staging system, which ignores evidence of nodal involvement, and the pathologic staging system, which places all surgically treated patients with nodal metastases in the stage IIIB category, has compounded the potential for misunderstanding.
Clinical Characteristics of the Primary Tumor
Tumor Diameter
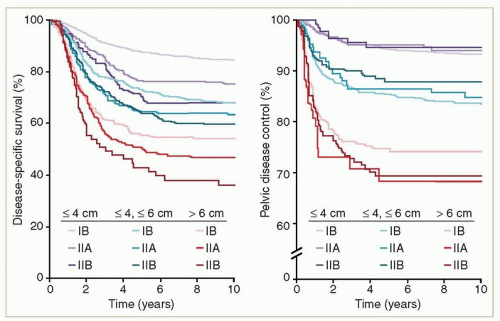
For patients treated with definitive RT, primary tumor diameter is probably the most important single predictor of central disease recurrence (
Fig. 10.1)
10; when the analysis is fully corrected for tumor diameter, other clinical features, such as paracervical involvement, appear to have little influence on local recurrence risk. Tumor diameter is also correlated with the presence of nodal metastases and with overall survival. For patients treated with primary surgery, both clinical tumor diameter and histologic measurement of tumor size are strong predictors of nodal metastasis and of disease recurrence.
Parametrial Involvement
Tumor can infiltrate the paracervical supporting ligaments by direct extension or the parametrial nodes by lymphatic spread. Ever since the FIGO staging system was devised in the early 20th century, that system has included the clinical impression of parametrial involvement as a dominant feature, at least in part because the presence of parametrial involvement has been considered a relative contraindication to primary surgical management. Parametrial involvement should probably still be considered a contraindication to surgery today. However, clinical assessment of parametrial involvement is notoriously inaccurate.
11,12,13 For example, in a large Austrian series of patients who had radical hysterectomy
for stage I to IIB cancer,
11 only 30% of patients diagnosed by physical examination as having “stage IIB” disease actually had histologic evidence of paracervical involvement; this was only slightly more than the 14% rate of parametrial involvement in patients with stage IB disease. Nodular endovaginal disease is commonly confused with parametrial involvement (
Chapter 4). Also, large tumors are more likely than smaller tumors to be labeled as stage IIB, confounding the correlation between parametrial involvement and survival. In a large study from MD Anderson,
10 the clinical impression of parametrial involvement did appear to be independently correlated with disease-specific survival but was a poor predictor of pelvic disease control (
Fig. 10.1). FIGO recently permitted MRI findings of parametrial involvement to be considered in the staging system. It is unclear whether MRI is more accurate than clinical examination in the evaluation of parametrial involvement, but MRI findings are likely to be somewhat more reproducible than findings on clinical examination.
Morphology
As cervical tumors enlarge, they may exhibit a predominantly exophytic, endocervical (“barrel-shaped”), or infiltrative growth pattern. MD Anderson studies have suggested that exophytic tumors are more responsive to RT and tend to have a lower rate of central disease recurrence than do endocervical tumors. In a 1994 study of patients with stage IB cancers, Eifel et al.
14 reported central disease recurrence rates of 3% for exophytic tumors 5 to 7.9 cm in diameter and 9% for predominantly endocervical tumors of similar size, although there was no difference in the overall rate of pelvic disease recurrence. This study predated the use of concurrent chemotherapy and RT (chemoradiation), which has reduced the overall incidence of central disease recurrence and may have blunted or eliminated the independent prognostic impact of morphology.
Pelvic Wall Involvement
Tumors that are fixed to pelvic wall structures are considered stage IIIB. These tend to be large, infiltrative cancers with a relatively poor prognosis. At MD Anderson, the prognosis of patients with stage IIIB disease is very similar to that of patients with earlier-stage cancers that measure 7 cm or more in diameter
10,15; both groups have 5-year disease-specific survival rates of 50% to 60%.
However, clinical assessment of pelvic wall fixation tends to be highly inaccurate. On the other hand, hydronephrosis, the second criterion for diagnosing stage IIIB disease, is a relatively objective finding. This provides a means for estimating physicians’ relative thresholds for interpreting physical findings as pelvic wall involvement—a relatively low proportion of stage IIIB patients having hydronephrosis suggests that physicians are more likely than others to diagnose a patient with questionable findings as having stage IIIB disease. For example, in a 2009 South Korean review, only 44 (11%) of 388 patients treated with radiation for stage IIIB disease between 1986 and 2004 had hydronephrosis.
16 In contrast, 165 (57%) of 287 patients with stage IIIB disease diagnosed at MD Anderson during these same years had hydronephrosis (unpublished data). Unfortunately, the rate of hydronephrosis is rarely reported in reports of locally advanced cervical cancer, making it difficult to estimate the pervasiveness of this cause of stage migration.
A number of factors contribute to overdiagnosis of pelvic wall involvement. Large tumors can fill the lower pelvis without extensively infiltrating adjacent tissues; these may seem immobile until initial response to treatment reveals that the paracervical tissues are free of gross disease. Also, prior pelvic infection can affect the mobility of the uterus. In our experience, MRI rarely demonstrates true direct invasion of lateral pelvic structures. Occasionally, cancer is seen directly invading from the cervix to the piriform muscle via the uterosacral ligaments; this is a poor prognostic sign, usually associated with very advanced disease.
Vaginal Involvement
Unless it is very minimal, vaginal involvement is a relative contraindication to primary surgical treatment because the chance of a positive margin is high. However, involvement of the upper two-thirds of the vagina generally has little independent influence on the prognosis of patients treated with radiation
10 (
Fig. 10.1). Most patients who have distal vaginal involvement also have massive primary tumors. Stage IIIA cancers that involve the distal vagina without pelvic fixation or hydronephrosis are rare. Some of these cancers are undoubtedly primary vaginal cancers that involved the cervix secondarily but are by convention classified as cervical cancers. Discontinuous involvement of the vagina is a poor prognostic sign,
17 but even patients with this finding can sometimes be cured
(CS 10.8).
Hydronephrosis
The finding of hydronephrosis is a poor prognostic sign because it is a sign of extracervical involvement associated with locoregionally advanced cervical cancer and because secondary renal failure can complicate treatment of cervical cancer. Although the relationship between hydronephrosis and prognosis is heavily confounded by other adverse findings, most investigators have found hydronephrosis to be an independent predictor of poor outcome. In our experience, patients with bilateral hydronephrosis do not have a worse prognosis than patients with unilateral hydronephrosis if outcome is corrected for other factors.
15Ureteral obstruction usually occurs at the base of the bladder as a result of local tumor infiltration. However, the ureters are also vulnerable to secondary involvement by lymph nodes, particularly in the region just distal to the common iliac bifurcation, where the ureters cross into the pelvis (
Fig. 5.2).
Acute or chronic renal compromise and, if treatment does not relieve the ureteral obstruction, long-term adverse effects
of urinary diversion can have a significant negative impact on quality of life and long-term survival.
Histologic Type
Squamous Carcinoma
Most primary cervical cancers are squamous carcinomas. Although squamous carcinomas are frequently subclassified according to the cell size, presence or absence of keratin, or degree of cell differentiation, these characteristics are poorly correlated with the likelihood of lymph node metastases and the likelihood of recurrence.
18
Adenocarcinoma
In the United States and most other economically advanced countries, ˜20% to 25% of cervical cancers are adenocarcinomas. This proportion has increased by about 30% since the 1970s, and the proportion of adenocarcinomas relative to squamous carcinomas has about doubled during that time.
19 The increase in adenocarcinomas relative to squamous carcinomas is in large part due to the fact that methods for detection of preinvasive adenocarcinomas are worse than methods for detection of squamous carcinomas.
20 Also, adenocarcinomas are significantly more likely to be associated with HPV-18 than are squamous carcinomas, and some studies have suggested that cytologic screening is less effective in the detection of HPV-18-positive cancers than in the detection of HPV-16-positive cancers.
20In general, although adenocarcinomas exhibit a number of morphologic patterns, the pattern does not appear to have a major independent influence on outcome.
21 However, adenocarcinomas differ from squamous carcinomas in that grade appears to be strongly correlated with the risk of recurrence for adenocarcinomas but not for squamous carcinomas. Adenosquamous cancers, which have both glandular and squamous elements, tend to have a particularly poor prognosis,
22 reflecting their high cytologic grade. Whether their prognosis is poorer than that of other high-grade adenocarcinomas is unclear. Serous cancers are rare but, in our experience, tend to exhibit an aggressive behavior similar to that of uterine serous cancers (
Chapter 13).
In a 1990 MD Anderson review of more than 367 patients treated with definitive radiation for cervical adenocarcinoma,
23 patients with stage II or III disease were found to have a much higher rate of pelvic and distant recurrence than was typical for squamous carcinomas treated at that institution; remarkably, 73% of patients with stage II adenocarcinoma in that study developed distant metastases. In a separate study of patients treated with radiation only for stage I cervical cancer, adenocarcinoma was found to be an independent risk factor for distant metastases, although the rates of pelvic disease recurrence were similar for squamous carcinoma and adenocarcinoma.
24 Adenocarcinoma may also to be a risk factor for local recurrence after radical hysterectomy, particularly for tumors of high grade with lymphovascular space invasion (LVSI).
23,25The prognostic differences between adenocarcinoma and squamous carcinoma appear to be blunted by the use of adjuvant treatments after hysterectomy
26 and by the use of concurrent chemoradiation.
27 For example, a subset analysis of the GOG-92 study suggested that patients with adenocarcinoma received a disproportionate benefit from adjuvant pelvic RT after radical hysterectomy.
26 However, in our experience, concurrent chemoradiation has not eliminated adenocarcinoma as a risk factor for recurrence of locoregionally advanced cervical cancer.
28Although the median time to recurrence of adenocarcinoma tends to be short,
22,23 in some cases, the disease can be very indolent, with pelvic disease recurrences not diagnosed until 5 years or more after RT.
23 In our experience, such late recurrences are extremely rare for squamous carcinoma.
Small Cell Neuroendocrine Cancer
Small cell neuroendocrine cancer is a rare subtype of cervical cancer that has a very high rate of distant metastasis and a poor prognosis even after aggressive multimodality treatment.
29,30 It should not be confused with small cell squamous carcinoma.
Lymph Node Metastasis
Lymph node metastasis is a common finding in patients with cervical cancer; 50% or more of patients with locally advanced disease have PET evidence of nodal metastases at diagnosis. The likelihood of lymph node involvement is correlated with tumor size, stage, and depth of invasion, and in surgically treated patients, the presence of LVSI is a strong predictor of lymph node involvement.
31The presence of lymph node metastases is correlated with increased rates of pelvic disease recurrence, distant metastases, and death from disease.
10,28,31 However, most patients with lymph node metastases at the time of their initial diagnosis of cervical cancer do not have evidence of synchronous distant metastases. The pattern of nodal metastasis tends to be orderly: paraaortic node metastases are rarely seen in the absence of iliac node metastases. The presence of paraaortic node metastases is associated with a poorer prognosis, although even patients with this finding can be cured in up to 50% of cases
32,33 (CS 10.5 to 10.10).
In a study of 256 patients with node-positive cervical cancer treated with radical hysterectomy and pelvic RT,
34 the rates of relapse and survival were independently correlated with the number of positive lymph nodes and with the presence of extracapsular extension but not with the size of the largest metastatic deposit; the authors did not specifically report the rates of pelvic disease recurrence.
FIGO stage remains only a very crude predictor of prognosis. Although the rigid rules for FIGO staging, if applied correctly, promote some level of international consistency in staging, these rigid rules are also a source of considerable frustration. Ultimately, FIGO stage, although somewhat useful for reporting and comparison of results, is of limited value
for treatment planning. The reporting of clinical findings according to the FIGO clinical staging system should always be supplemented with more detailed descriptions of clinical and tumor characteristics.
Risk Factors for Patients Undergoing Radical Hysterectomy
For patients undergoing radical hysterectomy, the finding of any type of extracervical involvement—lymph node metastases, parametrial involvement, or positive surgical margins—is associated with a high risk of pelvic disease recurrence.
12,13,31,35 However, because patients with such findings usually receive adjuvant treatment, the magnitude of the recurrence risk for patients with untreated extracervical involvement is difficult to estimate.
Local cervical tumor features are predictive of both lymph node involvement and outcome. In the GOG-49 study, a classic prospective registry study of patients undergoing radical hysterectomy for FIGO clinical stage I squamous carcinoma, depth of invasion, LVSI, and parametrial involvement were all strongly correlated with lymph node involvement.
36 For patients with involvement of the inner, middle, and outer thirds of the cervical stroma, the risks of lymph node involvement were 5%, 13%, and 26%, respectively; lymph node metastases were found in 25% of patients with LVSI versus 8% of patients without LVSI and 43% of patients with parametrial involvement versus 13% of patients without parametrial involvement. In a second analysis of the GOG-49 results,
31 the authors reported that clinical tumor diameter, depth of invasion, and LVSI were the most powerful predictors of relapse. The results of GOG-49 were refined such that various categories of tumor diameter, depth of invasion, and LVSI were combined to define a group of patients with an intermediate risk for recurrence after radical hysterectomy; these so-called Sedlis criteria were subsequently used to define eligibility for the GOG-92 study of adjuvant pelvic RT (
Table 10.3).
More recently, in a large Korean study, Ryu et al.
37 evaluated a variety of potential predictive models using development and validation groups. These authors confirmed the predictive value of the GOG-92 criteria but proposed a different model that was somewhat simpler and appeared to more accurately predict recurrence. In this model, patients who had any two of four findings—tumor diameter >3 cm, invasion of the outer third of the cervical stroma, LVSI, or adenocarcinoma—had a relative risk of recurrence 3.1 times that of patients without none or only one of those findings.