Cerebrovascular Disease: Introduction
Although cerebral vascular disease is the third leading cause of death in the United States after heart disease and cancer, it remains the single most important cause of disability. One of several pathologic processes that either lead to occlusion or rupture of an extra- or intracranial artery or vein produces the clinical manifestation of cerebral vascular disease in terms of primary ischemic stroke, transient ischemic attacks (TIAs), and/or primary hemorrhagic stroke. With either TIA or primary ischemic or hemorrhagic stroke, the prelude to acute, chronic, or preventive therapy is precise diagnosis. The diagnostic formulation must not only establish that the clinical entity is an ischemic stroke or TIA, or a hemorrhagic stroke, but also must localize and characterize the precise arterial or venous pathologic process causing the stroke, as well as elucidate the nature of the spared collateral circulation (Figures 64-1 and 64-2). This chapter focuses on identifying this pathological process as the pivot on which hinge rational medical and/or surgical treatments or preventive strategies. For both primary ischemic and/or primary hemorrhagic stroke, as for TIAs, the precise pathophysiologic process divides logically into specific stroke or TIA subtypes.
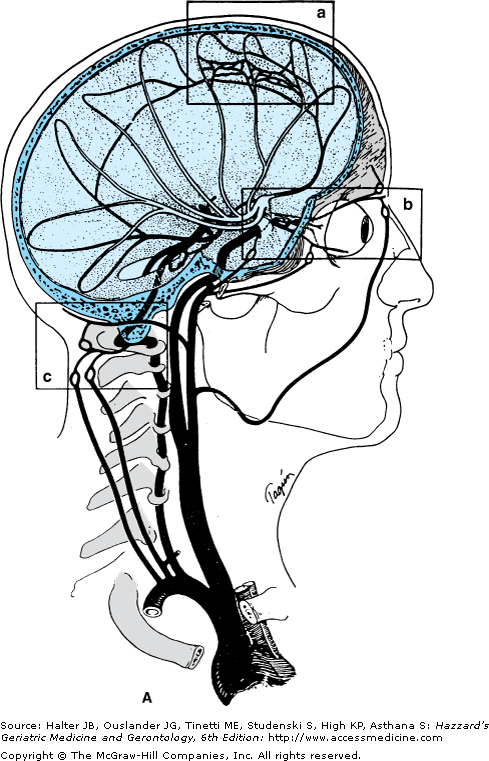
Figure 64-1.
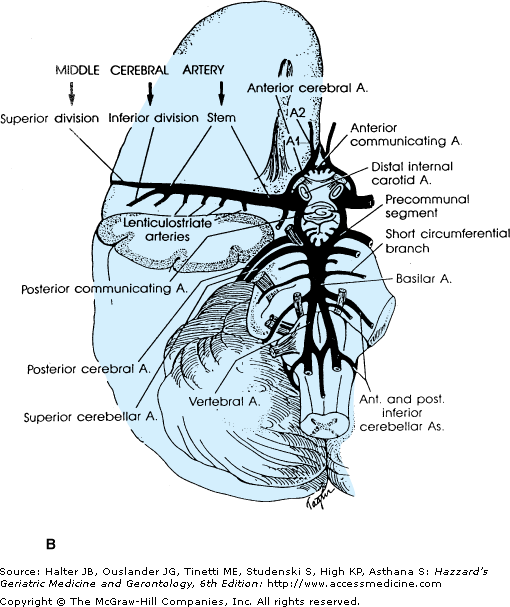
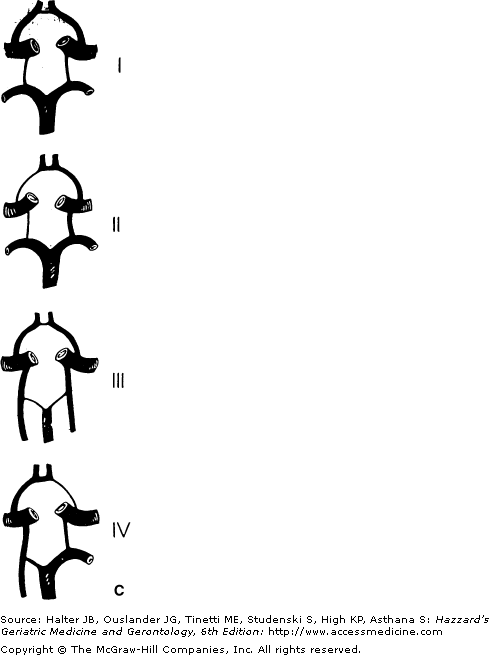
(A) Arrangement of the major arteries of the right side carrying blood from the heart to the brain. Also shown are vessels of collateral circulation that may modify the effects of cerebral ischemia (a, b, and c). Not shown is the circle of Willis, which also provides a source for collateral circulation. a. The anastomotic channels between the distal branches of the anterior and middle cerebral artery, termed borderzone or watershed anastomotic channels. Note that they also occur between the posterior and middle cerebral arteries and the anterior and posterior cerebral arteries. b. Anastomotic channels occurring through the orbit between branches of the external carotid artery and ophthalmic branch of the internal carotid artery. c. Wholly extracranial anastomotic channels between the muscular branches of the ascending cervical arteries and muscular branches of the occipital artery that anastomose with the distal vertebral artery. Note that the occipital artery arises from the external carotid artery, thereby allowing reconstitution of flow in the vertebral from the carotid circulation.(Part A courtesy of C. M. Fisher, MD.) (B) Diagram of the brainstem, cerebellum, inferior right frontal lobe, and temporal lobe transected. Principal branches of the vertebral basilar arterial system are pictured. Small branches of the vertebral and basilar artery that penetrate the medulla and pons are not pictured. The stem of the middle cerebral artery with its small, deep-penetrating lenticulostriate arteries and the circle of Willis with its small, deep-penetrating branches, are shown. (C). Roman numerals I, II, III, and IV represent some of the possible variations of the circle of Willis caused by atresia of one or more of its arterial components.
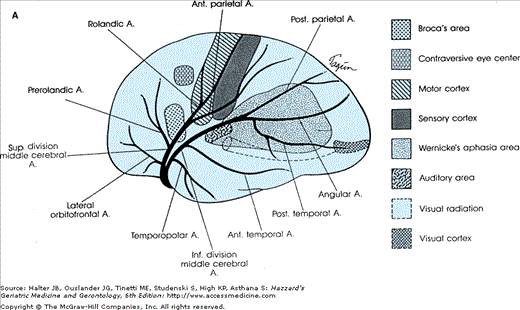
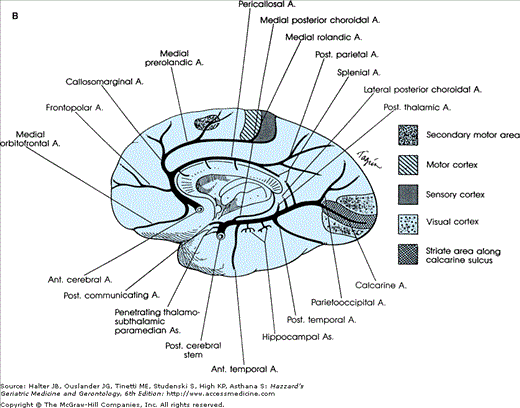
Figure 64-2.
(A) Diagram of a cerebral hemisphere, lateral aspect, showing the branches and distribution of the middle cerebral artery and the principal regions of cerebral localization. Note the bifurcation of the middle cerebral artery into a superior and inferior division. (B) Diagram of a cerebral hemisphere, medial aspect, showing the branches and distribution of the anterior cerebral artery and the principal regions of cerebral localization. (Courtesy of C. M. Fisher, MD.)
Ischemic Stroke or TIA Subtype
It is important to recognize that ischemic stroke and/or TIAs share the same pathophysiological causation in terms of the underlying arterial pathology. The terms “stroke” and “TIA” are no more a diagnosis that dictates a specific therapeutic strategy than are the terms “fever” and “abdominal pain.” Just as the primary care physician would treat the pneumococcal pneumonia giving rise to the fever, or the pancreatitis giving rise to abdominal pain, so should the underlying arterial pathophysiology of the ischemic stroke or TIA be the focus of a treatment strategy. The term “cerebral vascular accident” should be discarded and the terms “TIA” and “ischemic stroke” should be complemented by terms defining their pathophysiologic subtype. Primary ischemic stroke and TIA conveniently divide into four subtypes: (1) large artery atherothrombotic (15%); (2) embolic (57%—cardiac, ascending aorta, or unknown source); (3) small vessel lacunar (25%); and (4) other (3%), such as arterial dissection, venous sinus occlusion, and arteritis. The percentages of the various ischemic stroke or TIA subtypes or pathophysiologic cause identified in the National Institutes of Health (NIH) Stroke Databank vary with different ethnic population groups. African-Americans are at relatively higher risk of lacunar stroke and atherothrombotic stroke, particularly that portion of atherothrombotic stroke caused by intracranial arterial atherosclerosis.
When transient or sustained focal neurological symptoms or signs develop in a patient, the history, physical examination, and neurologic examination suggest that it is a stroke, and may even suggest the pathophysiological subtype. This presumed pathophysiologic TIA or stroke subtype diagnosis requires immediate noninvasive assessment of the extra- and intracranial arterial system, focusing on the arteries supplying the suspected symptomatic arterial territory. This includes the large arteries from the aortic arch to the locus of ischemia or infarct. The “parent” artery pathology, or lack thereof, then confirms the subtype, namely, large artery atherothrombotic, embolic, small vessel disease, or other. Often an embolus has fragmented and migrated, leaving the parent vessel patent, or it may have entered a branch of the parent vessel not shown in the scheme outlined in Figure 64-2. In that case, embolism is suggested only by the specific territory of the infarction. When a small infarction occurs in the territory of a single penetrating vessel deep in the brain (as might arise from the basilar artery, the middle cerebral artery stem, or the arteries of the circle of Willis), a small vessel lacunar stroke is diagnosed. To confirm the clinically suspected presumed pathophysiological diagnosis, computed tomography (CT) scanning alone in the acute or subacute phase is inadequate. CT has nearly perfect sensitivity for acute intracerebral hemorrhage (approaching 100%) and good sensitivity for acute subarachnoid hemorrhage (approximately 90%). Sensitivity for ischemic infarction in the acute setting is, however, much lower. Ischemic infarction may not be demonstrable by noncontrast CT for 12 to 14 hours after symptom onset; additionally, infarction involving only the cortical surface supratentorially, or infarction in the posterior fossa, can often be obscured by bone artifact. Therefore, contrast CT angiography, magnetic resonance imaging (MRI) with MR angiography, and/or carotid duplex Doppler and transcranial Doppler, should be obtained to outline the parent vessels (Figure 64-2), before specific treatment based on the pathologic process can be devised. Only with precise knowledge of the parent vessel pathology, or its absence, can therapy be properly addressed—whether it be thrombolytic, anticoagulant, or antiplatelet, interventional intra-arterial, or surgical. The following sections outline each of the four ischemic stroke and TIA subtypes, and intracerebral and subarachnoid hemorrhage, in terms of their pathophysiological process and clinical presentation. A discussion of a focused diagnostic approach to confirm that clinically presumed diagnosis follows. Based on the particular TIA or stroke subtype and its causative pathological process, acute, subacute, and preventive management strategies can then be addressed.
Atherothrombotic cerebral vascular disease accounts for only 15% of all ischemic infarcts in the NIH Stroke Databank. The atheromatous process occurs in strategically focal locations with predilection for four extra- and intracranial arterial locations (Figure 64-2): (1) the internal carotid artery origin, (2) or its siphon portion, (3) the middle cerebral stem, and (4) the vertebrobasilar junction. Although the origin of the common carotid artery and the vertebral arteries also are sites of atheromatous disease, they are less-often implicated in stroke or TIA. At each of the sites of predilection, two mechanisms are responsible for causing the stroke or TIA: (1) atherothrombotic plaque with clot formation that either occludes the vessel and gives rise to an embolic fragment that occludes a more distal vessel, that is, artery-to-artery embolus, or thrombus that propagates into a distal vessel; or (2) the atherothrombotic process might occlude or narrow the vessel to such a degree that distal flow is diminished and not compensated via the circle of Willis, that is, low-flow stroke or TIA. Generally, both artery-to-artery embolism and low-flow stroke occur when the vessel is narrowed by the atheromatous process to a degree that decreases pressure across the arterial segment, that is, producing a 70% or greater diameter stenosis, or frank occlusion. A TIA is said to occur if symptoms clear within an arbitrarily set time of 24 hours. In artery-to-artery embolic events, symptoms may last for shorter periods of time, and in low-flow transient ischemic events they may last only minutes to as long as an hour or more. Often, artery-to-artery or low-flow transient ischemic events may be associated with an MRI-proven area of infarction, notwithstanding resolution of the symptoms. When symptomatic stroke ensues in this setting, it is because the artery-to-artery embolic fragment lodges in an intracranial vessel with inadequate distal collateral supply, or because the clot propagates and occludes the vessel. A dramatic example of the latter occurs with internal carotid artery origin occlusion and propagation of the clot distally into the middle cerebral stem (Figures 64-1 and 64-2).
Low-flow stroke or TIA occurs less often from a cervical lesion because the circle of Willis provides distal collateral circulation. However, low-flow stroke or TIA occurs more often with atheromatous disease in the distal vertebral or in the basilar arteries. Similarly, the frequency of low-flow stroke or TIA increases when the atheromatous process is in the middle cerebral stem and compromises the ability of the circle of Willis to provide sufficient collateral flow. In 70% of the population, the circle of Willis is incompetent, with one or more of the connecting arteries atretic or functionally inadequate (Figure 64-2); in this circumstance, low-flow TIA or stroke may arise from atherothrombosis at the internal carotid artery origin or in its petrous or siphon portions.
Because of the advent of thrombolytic therapy, acute stroke diagnosis and management strategies differ significantly from those for subacute stroke or TIA. Acute treatment strategies for all subtypes of ischemic stroke are discussed at the end of this section. In subacute stroke or TIA, the evaluation and management are best directed in relation to the specific pathophysiological subtype, as outlined in the sections that follow.
Atherothrombotic Disease of the Anterior Cerebral Circulation (Origin of the Internal Carotid Artery, Its Major Branches, and the Common Carotid Artery)
In this arterial territory, atheroma occurs most often at the bifurcation of the common carotid artery, and usually begins on the posterior wall of the internal carotid artery origin.
- Internal carotid artery: Most often, atheroma at the origin of the internal carotid artery becomes symptomatic after it has narrowed the lumen by 70%, leaving a residual lumen diameter of 1.5 mm—the point where the pressure begins to drop across the stenosis, allowing both embolic or low-flow ischemic TIA and stroke to occur. Embolism from thrombus forming in an ulcerated crater may occur at 50% to 70% stenosis, but it is much less common, and very rarely occurs with lesser degrees of stenosis. Artery-to-artery embolism can also occur if the atheromatous process occludes the internal carotid artery origin forming a thrombus at the site. At times, the occluding thrombus may also propagate without embolization, reaching the ophthalmic artery origin and producing monocular blindness, or extending even more distally to the middle cerebral artery origin and producing a devastating, total, middle cerebral territory stroke.
The symptoms of the artery-to-artery embolism are variable, depending on the distal branch in which the embolism lodges, and the degree of distal collateral flow. The middle cerebral artery territory is the most often affected. The exact clinical manifestations depend on the precise location of the ischemia, whether in the territory of the ophthalmic artery, the middle cerebral artery, or the anterior choroidal artery. Transient monocular blurring or blindness, often with a shade lowering or telescopic effect, points to ischemia in the ophthalmic artery territory. Symptoms associated with ischemia in the other territories are discussed, in their respective sections, below.
Low-flow symptoms caused by internal carotid artery origin stenosis are less common than artery-to-artery embolism, and occur only if two conditions exist: (1) the lesion has to be hemodynamically significant, that is, severe enough to provoke a drop in pressure across the lesion, which is a situation supervening at a residual lumen diameter <1.5 mm and (2) collateral flow distally through the circle of Willis or external carotid artery ophthalmic routes is inadequate, leading to a low-pressure state either in the middle cerebral artery or in one or both of the anterior cerebral arteries. If there is internal carotid artery occlusion and little distal collateral flow then a complete middle cerebral syndrome may result. The posterior cerebral artery (PCA) territory may also be vulnerable to low flow when this artery arises directly from the internal carotid artery, that is, a “fetal PCA.” If the circle of Willis is complete, then occlusion of the internal carotid artery can be asymptomatic if it does not have an associated embolic or propagated thrombotic component.
Atheromatous disease in the internal carotid artery siphon occurs less often, but shares the same type of physiologic mechanisms for TIA and stroke as seen with atheromatous disease of the internal carotid artery origin. By contrast, middle cerebral stem atheromatous lesions mainly cause low-flow TIA or stroke. Emboli to the middle cerebral artery branches from that source occur less frequently and tend to be small. Common carotid origin stenosis rarely causes symptoms, but when they occur, they are most commonly caused by embolism to a distal intracranial artery.
- Middle cerebral artery: Middle cerebral artery territory symptoms may conveniently be divided into those involving the stem territory (M1 segment; see Figure 64-2) and those symptoms resulting from ischemia in the superior or inferior territory division or one of their cortical surface branches (Figures 64-1 and 64-2). When the stem of the middle cerebral artery is occluded by thrombosis of an ulcerated atheromatous plaque or artery-to-artery embolism, a complete middle cerebral artery syndrome may occur. It produces a complete hemiplegia equally involving the face, arm, hand, leg, and foot because of the ischemia in the small penetrating (lenticulostriate) arteries that arise from the middle cerebral stem supplying the basal ganglia and internal capsule. Eye deviation toward the ischemic hemisphere occurs when either hemisphere is affected. In addition, ischemia in the upper and lower division cortical surface branches of the middle cerebral artery gives rise to global or partial aphasia (dominant hemisphere) or to neglect and apractognosia (nondominant hemisphere). Which cortical surface structures are involved depends on the level of occlusion and degree of cortical surface collateral flow (Figure 64-1). Smaller emboli cause single superior or inferior branch of the middle cerebral artery syndromes, or partial branch syndromes. In the superior division, these might result in unilateral hand, hand and arm, or hand, arm, and shoulder weakness, unilateral face weakness, or expressive aphasia. Inferior division branch embolic syndromes include difficulty with reading, writing, auditory comprehension of language, or fluent aphasic speech with no limb weakness. Paraphasic errors abound in both inferior and superior division syndromes. Neglect of the left visual hemifield occurs most noticeably in nondominant syndromes, but may also occur on the right with dominant syndromes.
Low-flow syndromes occur when the internal carotid artery occlusion is not supported by adequate collateralization distally. They include recurrent transient brief episodes of hip and shoulder weakness, arm and leg weakness, speech trouble, and face or tongue numbness or weakness, many of which are similar, that is, stereotyped. Cortical surface infarcts secondary to low flow tend to occur in the distal field of the middle cerebral artery branches, before they anastomose with the anterior or posterior cerebral artery cortical surface branches, or in the distal field of the lenticulostriate penetrating arteries, affecting the deep white matter of the corona radiata.
- Anterior cerebral artery: Anterior cerebral arteries divide into two segments: part of the circle of Willis (A1 or stem) and the postcommunal (A2) segment distal to the anterior communicating artery (Figure 64-1). The A1 segment gives rise to several deep penetrating arteries that supply the anterior limb of the internal capsule, the anterior perforate substance, medullar anterior hypothalamus, and posterior part of the head of the caudate nucleus. Infarction in these territories is more often caused by an embolus than by local atheromatous disease. If the A2 segments of both right and left anterior cerebral arteries arise from a single anterior cerebral artery A1 segment, a normal anatomic variant, then symptoms occur in both hemispheres and include bilateral leg weakness. The most common symptoms of A2 segment occlusion are foot and leg weakness, and incontinence; occasionally—when both A2 segments are affected—abulia, gait apraxia, and forced grasping may also occur.
- Anterior choroidal artery: This artery arises from the internal carotid artery and supplies the posterior limb of the internal capsule and its posterolateral white matter, through which pass some of the geniculocalcarine fibers. The complete clinical syndrome consists of contralateral hemiparesis, hemianesthesia, and hemianopia. However, because this territory is also supplied by penetrating vessels of the middle cerebral artery stem and the posterior communicating and posterior choroidal arteries, syndromes with minimal deficits occur and patients frequently have an excellent recovery.
- Common carotid artery: All of the neurologic symptoms of internal carotid artery stenosis or occlusion can occur with common carotid artery thrombosis or an atherothrombotic lesion occurring at its origin. But the occurrence of transient ischemia or ischemia stroke arising from disease in the common carotid artery is far less than that of the internal carotid artery. Bilateral common carotid occlusions can cause faintness on arising, recurrent loss of consciousness, bilateral dim vision, headache, atrophy of the iris, pericapillary arteriovenous malformations, rubeosis iridis, optic atrophy, and claudication of the jaw muscles. They are associated with an incomplete aortic arch syndrome and have been associated with various combinations of carotid, subclavian, and innominate stenosis or occlusion.
Atherothrombotic Disease of the Posterior Cerebral Circulation: Vertebrobasilar and Posterior Cerebral Arteries and Their Branches
As seen in the anterior circulation, atherosclerosis has a predilection for certain parts of the posterior circulation—most frequently, the distal vertebral artery and the lower- to mid-basilar artery (Figure 64-1).
- Vertebral and posteroinferior cerebellar artery: An occlusion of the distal vertebral or its major branch, the posteroinferior cerebellar artery (PICA), may be caused by either atherothrombosis or by embolism from a proximal artery or the heart. An occlusion of either artery produces infarction in the lateral medulla. Atherothrombotic vertebral artery stroke is often heralded by TIA or minor stroke before the larger, completed stroke. The symptoms and signs vary, but the most frequent include vertigo, nausea and vomiting, hoarseness, dysphagia, ipsilateral facial numbness associated with impaired sensation of pain and heat over the ipsilateral face and contralateral arm and leg, ipsilateral Horner syndrome, and ipsilateral limb ataxia. The PICA also supplies the posteroinferior cerebellum that may become infarcted if collateral circulation from the superior cerebellar artery is inadequate. The infarct resulting from vertebral occlusion does not differ anatomically from that produced by PICA occlusion, except for a greater involvement of the restiform body in the latter. Nevertheless, involvement of the cerebellum does not appreciably alter the clinical picture, except in cases where cerebellar edema formation increases the pressure in the posterior fossa enough to result in progressive obtundation and death. Although cerebellar edema can be treated with osmotic agents such as mannitol, surgical decompression may be necessary.
- Basilar artery: TIA usually heralds atherothrombotic basilar artery occlusion and the consequent accompanying devastating brainstem infarction. The symptoms of a TIA in the territory of the distal vertebral and the basilar artery are more varied than in the carotid-middle cerebral territory because of the many different neuronal structures involved. Moreover, brainstem TIAs may be caused by disease of either of the small penetrating branches of the basilar or vertebral artery or of the basilar or vertebral arteries themselves. The penetrating branch disease may be atherothrombotic, involving the proximal origins of these small branch vessels, or lipohyalinotic, involving the small vessels deeper in the brainstem (see “Lacunar Stroke/TIA Subtype (Small Vessel Disease)” later in this chapter). In general, small vessel disease is less threatening than disease of the basilar trunk. Therefore, when brainstem TIA or acute stroke occurs, it is extremely important to determine whether the problem lies in the basilar artery or in one of its smaller branches. Disease of a basilar branch produces unilateral infarction, whereas disease of the basilar artery itself usually causes bilateral infarction. Transient dizziness associated with diplopia, dysarthria, and numbness around the mouth strongly indicate the presence of basilar insufficiency. Other important symptoms occurring less often include a general profound feeling of weakness of the entire body, staggering, and/or a feeling of propulsion. Bilateral signs such as gaze paresis or internuclear ophthalmoplegia associated with ipsilateral sensory loss or weakness signify ischemic infarction in both sides of the pons, and therefore exclude single penetrating branch disease as the culprit.
Many branch syndromes have been described; others await clinical correlation. Syndromes of unilateral brainstem infarction typically involve some combination of ipsilateral signs of the head and face, from involvement of cranial nerve nuclei or their fascicles, and contralateral signs in the limbs, from involvement of ipsilateral crossed long tracts, such as the corticospinal tract or spinothalamic tract. In both instances, that is, symptomatic disease of the basilar artery proper or its branches, the neurologic deficit may fluctuate, and usually is of low-flow type; artery-to-artery embolic TIA occur, but much less often, usually from embolism to the small basilar branches from a proximal or distal vertebral atheromatous lesion. In the case of an artery-to-artery embolic TIA or minor stroke, the events are single and the symptoms are less likely to fluctuate.
- Major basilar branches—anteroinferior cerebellar artery, superior cerebellar artery, posterior cerebral artery: These major artery branches of the basilar artery produce their own distinct pathophysiologic syndromes. They are most often caused by artery-to-artery embolism from an atherothrombotic source within the proximal basilar artery or the proximal or distal vertebral artery. Less often, an aortic or cardiogenic source is found. Rarely, primary atherothrombotic occlusion at their origins is the cause of the stroke or TIA.
- Superior cerebellar artery: Occlusion of the superior cerebellar artery results in one or more of the following symptoms: ipsilateral cerebellar ataxia (caused by ischemia of the middle and/or upper cerebellar peduncle, or dentate nucleus); nausea and vomiting; dysarthria and contralateral loss of pain and temperature sensation over the extremities, body, and face (caused by ischemia of the spinal and trigeminal thalamic tract); and ataxic tremor of the ipsilateral upper extremity. Horner syndrome, partial deafness, and palatal myoclonus may occur rarely. Partial syndromes occur frequently.
- Anteroinferior cerebellar artery: Occlusion of the anteroinferior cerebellar artery produces variable degrees of infarction because the size of this artery varies inversely with that of the PICA. The territory it supplies usually includes the lateral midpons, middle cerebellar peduncle, cerebellum, and the labyrinth and cochlea. The principal symptoms may include ipsilateral deafness, facial weakness, vertigo (whirling dizziness), nausea, vomiting, nystagmus, tinnitus, deafness, cerebellar ataxia, Horner syndrome, and paresis of conjugate lateral gaze. The opposite side of the body loses pain and temperature sensation. An occlusion close to the origin of the artery may cause cortical spinal tract signs.
- Paramedian and short circumferential branches of the basilar artery: Occlusion of one of the 5 to 7 short circumferential branches of the basilar artery affects the lateral two-thirds of the pons and/or middle or superior cerebellar peduncle, whereas occlusion of one of the 7 to 10 paramedian branches of the basilar artery affects a wedge-shaped area on either side of the medial pons. Many brainstem syndromes with cranial nerve abnormalities and crossed hemiplegia have been given eponyms; others await description.
- Posterior cerebral artery: Arising from the bifurcation of the top of the basilar artery, each posterior cerebral artery divides into two segments, each with its distinct clinical pathophysiologic syndrome: (1) the proximal precommunal segment, beginning at the top of the basilar artery and going to the posterior communicating artery takeoff, gives penetrating branches to the subthalamus, thalamus, and midbrain, (2) the postcommunal segment, beginning at the posterior communicating artery takeoff, supplies the medial inferior temporal lobe and the medial occipital lobe distally. Twenty percent of the time, one or both of the right or left posterior cerebral artery precommunal (P1) segments are atretic. In that case, the postcommunal (P2) segment is then supplied by the internal carotid artery via the posterior communicating artery. The majority of ischemic syndromes, TIA, or stroke, result from embolism (artery-to-artery, cardioaortic, or unknown source) to the pre- and postcommunal segments and/or one of their branches. Primary atherothrombotic disease of the posterior cerebral artery is much less common.
- Precommunal posterior cerebral artery syndromes include midbrain, subthalamic, and thalamic signs that vary depending on whether the embolus occludes the top of the basilar area, the right or left posterior cerebral artery precommunal segment, that is, the stem, or the penetrating artery branches of the precommunal segment. Coma and quadriplegia, resulting from infarction of the reticular activating system and bilateral corticospinal tracts within the midbrain, are the consequence of a devastating top of the basilar occlusion. But, otherwise, branch syndromes abound, usually with third nerve and contralateral motor or sensory findings. There is a normal anatomic variant in which a single large medial mesencephalic artery, the artery of Percheron, penetrates upward to supply both sides of the subthalamus as well as part of the midbrain. Occlusion of this artery results in bilateral ptosis, paralysis of upgaze, and decreased consciousness, caused by involvement of the periaqueductal gray and the mesencephalic reticular formation. When only a single penetrating precommunal artery territory is involved, small vessel lacunar disease results (see “Lacunar Stroke/TIA Subtype (Small Vessel Disease)” later in this chapter).
- Postcommunal posterior cerebral artery syndromes include cortical branches to the medial inferior temporal lobe, giving rise to memory loss and delirium, and branches to the medial occipital lobe, giving rise to homonymous visual field defects. Distal field border zone ischemia of the posterior cerebral and middle cerebral arteries give rise to visual impairment syndromes that include inability to recognize faces or pictures or to put items in a picture together to form an object (Balint syndrome).
- Precommunal posterior cerebral artery syndromes include midbrain, subthalamic, and thalamic signs that vary depending on whether the embolus occludes the top of the basilar area, the right or left posterior cerebral artery precommunal segment, that is, the stem, or the penetrating artery branches of the precommunal segment. Coma and quadriplegia, resulting from infarction of the reticular activating system and bilateral corticospinal tracts within the midbrain, are the consequence of a devastating top of the basilar occlusion. But, otherwise, branch syndromes abound, usually with third nerve and contralateral motor or sensory findings. There is a normal anatomic variant in which a single large medial mesencephalic artery, the artery of Percheron, penetrates upward to supply both sides of the subthalamus as well as part of the midbrain. Occlusion of this artery results in bilateral ptosis, paralysis of upgaze, and decreased consciousness, caused by involvement of the periaqueductal gray and the mesencephalic reticular formation. When only a single penetrating precommunal artery territory is involved, small vessel lacunar disease results (see “Lacunar Stroke/TIA Subtype (Small Vessel Disease)” later in this chapter).
- Superior cerebellar artery: Occlusion of the superior cerebellar artery results in one or more of the following symptoms: ipsilateral cerebellar ataxia (caused by ischemia of the middle and/or upper cerebellar peduncle, or dentate nucleus); nausea and vomiting; dysarthria and contralateral loss of pain and temperature sensation over the extremities, body, and face (caused by ischemia of the spinal and trigeminal thalamic tract); and ataxic tremor of the ipsilateral upper extremity. Horner syndrome, partial deafness, and palatal myoclonus may occur rarely. Partial syndromes occur frequently.
As defined by C. Miller Fisher through fastidious brainstem serial section, lacunar infarct results from an occlusion of a small single penetrating artery arising from the circle of Willis, the middle cerebral stem, the basilar artery, or either distal vertebral arteries. These penetrating arteries vary in size from 100 to 400 microns in diameter, rendering the infarct size finite from 0.5 to 1.5 cm in diameter, and usually no more than 3 cm in the longest dimension. The cause is lipohyalinotic narrowing or occlusion in the mid- or distal part of the artery or atherothrombotic lesion at its origin; embolism is rarely the cause. Lacunar strokes account for 25% of all strokes in the NIH Stroke Databank. These strokes cause recognizable clinical syndromes that evolve over hours to days, and may be preceded by transient symptoms (lacunar TIAs). The location of the ischemia determines the nature and severity of the symptoms. Recovery occurs often within days, but in some with especially strategically placed infarcts, significant disability is persistent.
The most common lacunar syndromes are the following:
- Pure motor hemiparesis from an infarct in the posterior limb of the internal capsule or basis pontis. The face, arm, leg, foot, and toes are equally paretic or plegic, but with no sensory deficit. The weakness may be intermittent (TIA), progress in a stepwise manner, or appear abruptly. The progression may result in a complete hemiplegia. Improvement almost to normal occurs in many cases.
- Pure sensory stroke from an infarct in the ventrolateral thalamus. This type of infarct produces face, arm, and leg sensory involvement with numbness, tingling, and loss of pain and temperature. The patient generally recovers but often is left with a sensory sensation that is abnormal. On rare occasions, an intolerable pain syndrome with dysesthesia occurs in the involved extremities some months afterwards (Dejerine–Roussy syndrome).
- Ataxic hemiparesis from an infarct in the basis pontis caused by an infarct in the basis pontis or corona radiata near the genu of the internal capsule.
- Dysarthria–clumsy hand syndrome caused by lacunar infarction of the genu of the internal capsule or, less frequently, the corona radiata or the paramedian rostral pons, with resulting mild contralateral arm ataxia or arm weakness, and dysarthric speech.
When multiple, lacunar infarctions may produce bilateral pyramidal and corticobulbar motor system signs, dysarthria, and a slowed abulic mental state with emotional lability, that is, pseudobulbar palsy. Such presentations have, however, become rare in today’s era of effective antihypertensive therapy.
Careful attention to antihypertensive, diabetic, and dyslipidemic therapies offer the best form of prevention. In all lacunar stroke or TIA, it is essential to ascertain that the large arteries at the base of the brain giving rise to the penetrating small arteries, that is, the basilar artery, the middle cerebral artery stem, or the posterior cerebral artery stem, are not, in themselves, harboring a thrombus threatening their occlusion and causing the lacunar syndrome (see “Evaluation and Therapeutic Strategies for Ischemic Stroke” below).
In this pathophysiologic stroke/TIA subtype the embolic fragment occluding the intracranial vessel, leading to the ischemia or infarction is derived from the aortic arch, the heart, or an unknown source (cryptogenic stroke), rather than from a local arterial source in the extra- or intracranial arteries, such as atherothrombosis or dissection.
Embolic strokes are diagnosed when a sudden or stuttering neurologic deficit appears in the territory of a large intracranial artery at the base of the brain or in one of its major branches, and the extra- or intracranial arterial supply to the ischemic zone does not have a detectable significant stenotic or thrombotic occlusive lesion as detected by ultrasonography, MRI, or CT angiographic imaging. In some cases, the embolic fragment may be seen, on imaging, to occlude the vessel or a distal branch. In other cases, the suspected embolic material is not visualized because it has already been dissolved by the endogenous fibrinolytic system, but not before significant ischemia has occurred. When the above definition of embolic stroke is used, it accounts for fully 60% of all strokes in the NIH Stroke Databank.
It is most convenient, for determining appropriate preventive therapy, to divide these types of emboli into treatment categories. First, those commonly accepted cardiac source emboli where anticoagulant therapy is standard of practice. Second, commonly accepted cardiac source emboli where anticoagulant therapy is contraindicated, as in bacterial endocarditis or atrial myxoma. Third, those possible cardioaortic sources that can only be diagnosed by transthoracic echocardiogram or transesophageal echocardiogram, in which debate exists as to the best therapy, be it anticoagulant, antiplatelet, or cardiointerventional. Fourth, those truly cryptogenic strokes in which a possible or definite cardiac and aortic source is excluded (Table 64-1). Within all of the source categories above, the level of activity of the hemostatic system may be a significant determinant in the incidence of local thrombus formation and embolism. Therefore, in addition to careful evaluation of the heart, evaluation of the hemostatic system, as well as exclusion of a primary arterial source of embolism, is essential in order to plan appropriate pathophysiology-based treatment.
Cardiac source is definite: anticoagulant therapy generally considered standard of practice. |
Left ventricular thrombi |
Left atrial thrombi |
Rheumatic valvular disease |
Mechanical prosthetic valves |
Atrial fibrillation |
Nonbacterial thrombotic endocarditis |
Cardiac source is definite: anticoagulation considered hazardous. |
Bacterial endocarditis |
Atrial myxoma |
Cardiac source is possible: synonyms in the literature include “unknown source,” “cryptogenic stroke”—these diagnoses are made by transthoracic or transesophageal echocardiogram. The efficacy of antiplatelet versus anticoagulant therapy is uncertain. |
Mitral annular calcification |
Left ventricular dysfunction and dilated cardiomyopathy |
Postmyocardial infarction with or without left ventricular aneurysm or thrombi |
Left atrial spontaneous echo contrast |
Patent foramen ovale |
Atrioseptal aneurysm |
Valvular strands |
Ascending aortic atheromatous disease: mobile plaque 4 mm or greater. |
Truly unknown-source embolic stroke. |
The clinical presentation of embolism in the anterior and posterior cerebral circulation is similar to that of artery-to-artery embolism, and was discussed in the preceding section. The nature and severity of the symptoms depend entirely on the location of the embolic fragment occluding the artery and the spared collateral circulation to its cerebral territory. The artery occluded, in turn, depends on the size of the embolic fragment and the extracranial artery it enters. In the case of an embolic fragment ending in the vertebral artery, it may migrate, stop, and then migrate again, leaving a trail of infarcted tissue from the cerebellum and lower brainstem to the upper brainstem, thalamus, and temporal and occipital lobes. Embolic fragments that enter the middle cerebral stem often disperse to branches of the superior and inferior division of the middle cerebral artery, giving rise to a patchy infarct in the deep or basal lenticulostriate arterial territory, and in the cortical surface branch territories.
In every case of embolic stroke, embolism from an infected heart valve should be considered because of the devastating consequences if not detected. Subacute bacterial endocarditis may have a very subtle presentation. A normal erythrocyte sedimentation rate at the time of the initial evaluation is a quick means of excluding it.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree