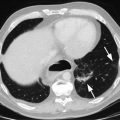
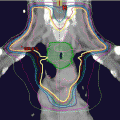
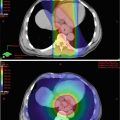
Fig. 2.1
Histological features of esophageal cancer prepared by formalin fixation and paraffin embedding reveal a well-differentiated squamous cell carcinoma with good histological detail
In recent years, the use of tissue microarray (TMA) has increased for testing molecular markers in large numbers of samples by either immunohistochemistry or FISH (Fig. 2.2). In the TMA technique, a hollow needle is used to remove tissue cores as small as 0.6 mm in diameter from regions of interest in paraffin-embedded tissues. These tissue cores are then inserted in a recipient paraffin block in a precisely spaced array pattern.
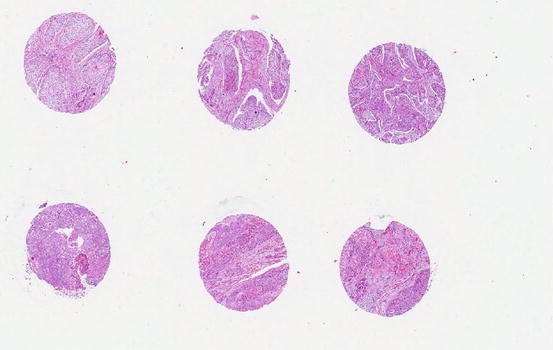

Fig. 2.2
Tissue microarray of esophageal squamous cell carcinoma showing multiple cores of cancer tissue in a block to allow testing of molecular markers simultaneously in many cases in one experimental run
The drawback of working on paraffin-embedded tissues is that formalin irreversibly cross-links proteins via the amino groups, thus preserving the structural integrity of the cells so they can be stained with dyes used to analyze for abnormalities in the tissue that indicate cancer. The effect of these cross-linking fixatives on the nucleic acids and proteins may impair the molecular interactions. In recent decades, esophageal cancer tissues have been prospectively collected by snap freezing in liquid nitrogen and storage at −80 °C. The collection included substantial clinical and scientific effort and provided tissues that are superior in quality for molecular studies. On the other hand, the morphological features are inferior to those obtained using paraffin-embedded sections (Fig. 2.3).
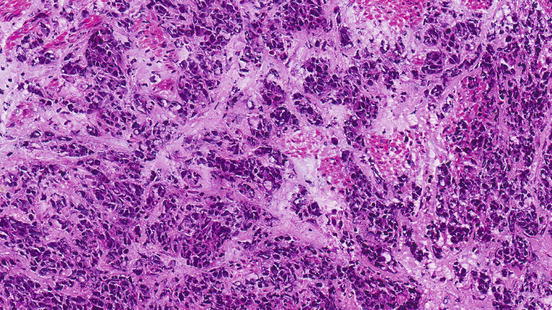

Fig. 2.3
Histological features of esophageal cancer prepared by sectioning of frozen tissues showing inferior histological detail when compared to Fig. 2.1
It is worth noting that research using this approach cannot provide functional dynamic studies of esophageal cancers.
Cancer Cell Lines
For functional studies in esophageal cancer, studies can be performed in cancer cell lines derived from tissues obtained freshly from surgery. Cancer cell lines need proper medium to grow. Cancer cell lines often grow without attaching to a surface, and they can proliferate to a very much higher density in a culture dish. The resulting transformed cancer cell lines, in reciprocal fashion, can often cause tumors if injected into a susceptible animal to generate an animal model. Cancer cells can be harvested from the animal and form a more stable cancer cell line. In esophageal cancers, some of the commonly used cell lines are actually secondary cell lines. Cancer cell lines can allow functional studies to be performed. They can be stored in liquid nitrogen for an indefinite period and retain their viability when thawed.
In esophageal cancers, there are published cancer cell lines available for both adenocarcinoma and squamous carcinoma [76–79]. When compared to esophageal squamous cell carcinoma, esophageal adenocarcinoma is more uniform in characteristics as the risk factors and pathogenesis are more established. Model research on esophageal adenocarcinoma relies almost entirely on a relatively small set of established cancer cell lines. The high genomic similarities between the esophageal cell lines and their original cancers provide rationale for their use. Nonetheless, cancer cell lines nearly always differ in important ways from the original cancer from which they were derived.
Animal Models
Animal models are important to study the effects of cancer in vivo and also for the production of cancer cell lines. In more clinical relevant applications, an animal model is a must for developing therapeutic strategies. Cancer development is a complex process with the accumulation of genetic alterations and their downstream effects as well as interactions with the microenvironment in different tissues. The cancer microenvironment and its interactions with the cancer are important in determining the growth dynamics of different cancers.
Injection of cancer or cancerous cells in the subcutaneous tissue of the skin of immunodeficient mice is a common practice to produce a cancer model in animals (Fig. 2.4). In esophageal cancers, this approach cannot recapitulate the microenvironment of the esophagus or the response to the targeting carcinogens. The current approach is to make an orthotopic (occurring at normal place) model for both esophageal squamous cell carcinoma and esophageal adenocarcinoma [80, 81]. The orthotopic model provides the most optimum environment for cancer growth and drug testing. In the anatomical setting of esophageal cancer, the site is very difficult to approach surgically. Different approaches have been applied but many of these have some shortcomings. The establishment of these orthotopic models needs to involve radiological guidance (magnetic resonance imaging and fluorescence imaging) so the cancer and the metastases can be visualized in real time [82]. In addition, pathological examination is important to clarify the histological typing, microscopic location, and the microenvironment of the cancer in the animal [83].
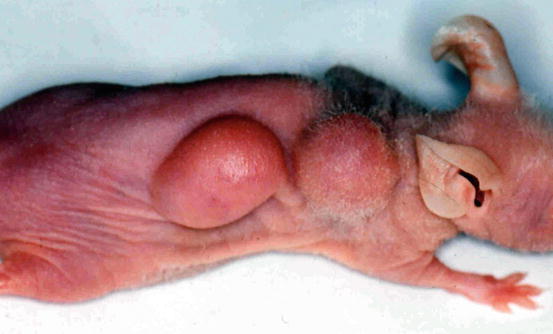

Fig. 2.4
Tumor produced in an immunodeficient mouse after subcutaneous injection of primary esophageal squamous cell carcinoma
References
1.
Lam KY, Ma L. Pathology of esophageal cancers: local experience and current insights. Chin Med J. 1997;110:459–64.PubMed
2.
3.
4.
5.
Lam KY, Dickens P, Loke SL, Fok M, Ma L, Wong J. Squamous cell carcinoma of the esophagus with mucin-secreting component (mucoepidermoid carcinoma and adenosquamous cell carcinoma): a clinicopathologic study and a review of literature. Eur J Surg Oncol. 1994;20:25–31.PubMed
6.
Law SYK, Fok M, Lam KY, Loke SL, Ma LT, Wong J. Small cell carcinoma of the esophagus. Cancer. 1994;73:2894–9.PubMed
7.
8.
Lam KY, Law S, Tung PH, Wong J. Esophageal small cell carcinoma: clinicopathologic parameters, p53 overexpression, proliferative marker, and their impact on pathogenesis. Arch Pathol Lab Med. 2000;124:228–33.PubMed
9.
Garbuglia AR. Human papillomavirus in head and neck cancer. Cancers (Basel). 2014;6:1705–26.CrossRef
10.
11.
Westra WH. Detection of human papillomavirus (HPV) in clinical samples: evolving methods and strategies for the accurate determination of HPV status of head and neck carcinomas. Oral Oncol. 2014;50:771–9.PubMedCentralCrossRefPubMed
12.
He D, Zhang DK, Lam KY, Ma L, Ngan HYS, Liu SS, Tsao SW. Prevalence of HPV infection in esophageal squamous cell carcinoma in Chinese patients and its relationship to the p53 gene mutation. Int J Cancer. 1997;72:959–64.PubMed
13.
14.
15.
Löfdahl HE, Du J, Näsman A, Andersson E, Rubio CA, Lu Y, Ramqvist T, Dalianis T, Lagergren J, Dahlstrand H. Prevalence of human papillomavirus (HPV) in oesophageal squamous cell carcinoma in relation to anatomical site of the tumour. PLoS One. 2012;7, e46538.PubMedCentralCrossRefPubMed
16.
17.
18.
Lam KY, Srivastava G, Leung ML, Ma L. Absence of Epstein-Barr virus in esophageal squamous cell carcinoma: a study of 74 cases using in-situ hybridization. J Clin Pathol Mol Pathol. 1995;48:M188–90.CrossRef
19.
Wang ZK, Yang YS. Upper gastrointestinal microbiota and digestive diseases. World J Gastroenterol. 2013;19:1541–50.PubMedCentralCrossRefPubMed
20.
Gibson MK, Dhaliwal AS, Clemons NJ, Phillips WA, Dvorak K, Tong D, Law S, Pirchi ED, Räsänen J, Krasna MJ, Parikh K, Krishnadath KK, Chen Y, Griffiths L, Colleypriest BJ, Farrant JM, Tosh D, Das KM, Bajpai M. Barrett’s esophagus: cancer and molecular biology. Ann N Y Acad Sci. 2013;1300:296–314.CrossRefPubMed
21.
Lam KY, Tsao SW, Zhang D, Law S, He D, Ma L, Wong J. Prevalence and predictive value of p53 mutation in patients with esophageal squamous cell carcinomas: a prospective clinicopathological study and survival analysis of 70 patients. Int J Cancer. 1997;74:212–9.PubMed
22.
Appelman HD, Matejcic M, Parker MI, Riddell RH, Salemme M, Swanson PE, Villanacci V. Progression of esophageal dysplasia to cancer. Ann N Y Acad Sci. 2014;1325:96–107.CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree