The pathophysiology of vasoocclusion is thought to involve a wide variety of adhesive interactions involving erythrocytes, leukocytes, and the endothelium. Selectins are expressed by leukocytes, platelets, and the endothelium, among other tissues. They contribute to a wide variety of physiologically important cell-cell interactions, including adhesion of all types of blood cells to the endothelium. In vitro, in vivo, and early-phase clinical studies suggest that E-selectin and pan-selectin inhibitors may be promising new therapeutic agents for the treatment of vasoocclusion in sickle cell disease.
Key points
- •
Adhesion of sickle red blood cells (RBCs) and leukocytes to endothelium, as well as to each other, is critical to the process of vasoocclusion in sickle cell disease (SCD).
- •
Selectins mediate rapidly reversible adhesive interactions, leading to rolling and tethering of cells under conditions of shear stress. This type of transient slowing or immobilization can lead to integrin activation and firm integrin-mediated adhesion.
- •
Both in vitro and in vivo preclinical studies support the hypothesis that E-selectin-mediated interactions have a critical role in vasoocclusion in SCD.
- •
Early-phase clinical studies suggest that an investigational drug, GMI-1070, which is a pan-selectin inhibitor with most activity against E-selectin, may be an effective intervention capable of shortening time to resolution of vasoocclusion in SCD.
Introduction
Sickle cell disease is characterized by episodic, acutely painful vasoocclusive episodes. The pathophysiology of vasoocclusion is thought to involve a wide variety of adhesive interactions involving erythrocytes (RBCs), the endothelium, and leukocytes, including neutrophils, monocytes, and lymphocytes. Platelets likely also contribute to the vasoocclusive process that characterizes SCD ( Fig. 1 ).
All hematopoietic cells, as well as endothelial cells, express multiple adhesion molecules, and many of these have been demonstrated in a variety of in vitro, in vivo, and ex vivo model systems to play a role in adhesive interactions that occur as a result of the presence of sickle RBCs (SS RBCs). Because such adhesive events are believed to be critical to the vasoocclusive process, they are a particularly attractive therapeutic target by which to address the cause of greater than 90% of health care required by patients with SCD. Most patients with SCD experience at least one such acutely painful episode annually, and many experience multiple events each year. Each event typically requires parenteral opioid therapy, often during a hospital stay of many days. However, identifying the most important adhesion receptors to target with potentially therapeutic drugs has been challenging.
Introduction
Sickle cell disease is characterized by episodic, acutely painful vasoocclusive episodes. The pathophysiology of vasoocclusion is thought to involve a wide variety of adhesive interactions involving erythrocytes (RBCs), the endothelium, and leukocytes, including neutrophils, monocytes, and lymphocytes. Platelets likely also contribute to the vasoocclusive process that characterizes SCD ( Fig. 1 ).
All hematopoietic cells, as well as endothelial cells, express multiple adhesion molecules, and many of these have been demonstrated in a variety of in vitro, in vivo, and ex vivo model systems to play a role in adhesive interactions that occur as a result of the presence of sickle RBCs (SS RBCs). Because such adhesive events are believed to be critical to the vasoocclusive process, they are a particularly attractive therapeutic target by which to address the cause of greater than 90% of health care required by patients with SCD. Most patients with SCD experience at least one such acutely painful episode annually, and many experience multiple events each year. Each event typically requires parenteral opioid therapy, often during a hospital stay of many days. However, identifying the most important adhesion receptors to target with potentially therapeutic drugs has been challenging.
Selectins and selectin-mediated adhesion
Among the adhesion receptors expressed by both hematopoietic and endothelial cells are the 3 known types of selectins: P-selectin, E-selectin, and L-selectin. Selectin ligands comprise a variety of sialylated and fucosylated carbohydrates containing an epitope common to both sialyl-Lewis a (sialyl-Le a ) and sialyl-Lewis X (sialyl-Le x ). Selectins contribute to a wide variety of physiologically important processes, including interactions of hematopoietic stem cells with the bone marrow microenvironment, homing of lymphocytes to high endothelial venules, migration of leukocytes to areas of inflammation, and metastasis of cancer cells.
In general, selectins mediate rapid on-off interactions, whereas integrins, another class of ubiquitous adhesion receptors, mediate high-affinity, stable adhesion. Selectins are therefore thought to provide the initial interaction between hematopoietic cells in motion and other cells, which may be stationary (such as endothelial cells) or also in motion, such as other circulating blood cells. Shear stress may be critical to the activation of at least some selectin-mediated events, which then lead to “tethering,” or short-lived adhesion of one cell to another. In interactions involving endothelial cells, “rolling” is then observed, consisting of repeated short-lived interactions of hematopoietic cells in motion with the stationary surface of the endothelium. Ultimately, these transient interactions are followed by firmer cell-cell adhesion provided by other adhesion receptors and ligands. Thus, selectins are responsible for rolling of circulating cells on endothelial surfaces, while integrins and some other adhesion receptors are responsible for more permanent capture of such cells.
Structural Characteristics of Selectins
The 3 species of selectin compose a family of adhesion receptors with similar structures but variable size and ligand specificity ( Fig. 2 ). The region responsible for binding carbohydrate ligands is at the N-terminus and is a lectin domain largely dependent on calcium for its activity. Further toward the membrane is the epidermal growth factor (EGF) domain, which is linked to consensus repeats. The number of consensus repeats is unique to each selectin, ranging from 2 in L-selectin to 6 in E-selectin and 9 in P-selectin. The rest of the molecules consist of a transmembrane domain and short cytoplasmic domain. All 3 selectin molecules share about 60% homology in their lectin and EGF domains.
E-Selectin
E-selectin, also known as CD62E, endothelial-leukocyte adhesion molecule 1, or leukocyte-endothelial cell adhesion molecule 2, is encoded by the SELE gene, which is on chromosome 1 near the gene encoding L-selectin. E-selectin is primarily expressed by activated endothelial cells. It is poorly expressed by resting endothelial cells; it is also expressed in skin and bone marrow. In general, E-selectin is expressed after inflammatory stimuli induce expression of SELE ; thus, E-selectin expression requires de novo protein synthesis and takes 3 to 5 hours to appear after stimulation, peaking up to 12 hours after exposure to an appropriate cytokine. Exposure to shear stress also affects E-selectin expression, with high shear stress increasing E-selectin expression.
Both polymorphonuclear and mononuclear leukocytes express E-selectin ligands and use them in their interactions with the endothelium during inflammation.
L-Selectin
L-selectin (CD62L) is a constitutively expressed selectin, present on subsets of T lymphocytes, including both naive and central memory T cells. L-selectin is also expressed by neutrophils and monocytes. Thus, it can play a broad role in leukocyte trafficking. Expression of L-selectin by lymphocytes and neutrophils is regulated by the action of endometalloproteases that cleave L-selectin from the cell surface when cells are activated. Loss of L-selectin is normally accompanied by increased expression of other leukocyte adhesion receptors. As L-selectin is cleaved from leukocyte surfaces, the level of plasma-soluble L-selectin (sL-selectin) increases. Soluble L-selectin may play a role in downregulation of leukocyte adhesion, as it retains biological activity and thus may block leukocyte adhesion generally. Ligand binding to cell surface L-selectin has been shown to set off a signaling cascade involving src-tyrosine kinase p56 lck , Ras, mitogen-activating protein kinase, and Rac2, leading to increased synthesis of O 2 − . On the endothelial side, L-selectin-mediated adhesion depends on activation of the endothelium, as endothelial cells express little ligand without activation.
Characteristics of P-selectin are discussed in an article by Kutlar and Embury elsewhere in this issue.
Preclinical studies of the role of E- and L-selectins in SCD
Both P-selectin and E-selectin have been shown to contribute to adhesive events in in vitro models of SCD. They seem to play critical roles in vasoocclusion in animal models of SCD, and reagents that block P-selectin- and E selectin-mediated interactions have been demonstrated to prevent or ameliorate vasoocclusion in experimental models. The role of L-selectin in SCD is less clear.
L-Selectin in Sickle Cell Disease
Several studies have shown that neutrophils are activated in SCD, even at steady state. Although neutrophil activation in SCD is widely recognized, expression of L-selectin by neutrophils in SCD has been variably described as both increased and decreased. Lard and colleagues found a significant decrease in L-selectin expression by neutrophils in steady state SCD, as well as increased levels of plasma sL-selectin. This observation is consistent with the view that neutrophils decrease expression of cell-surface L-selectin in response to activation. These investigators also found that this decrease was significantly more pronounced during vasoocclusive episodes. Other neutrophil markers were also consistent with neutrophil activation. However, Okpala and colleagues described SCD neutrophils and lymphocytes as having increased L-selectin expression compared with HbAA controls and reported that monocyte L-selectin expression increased during vasoocclusion. This group also suggested that subjects with higher leukocyte L-selectin and integrin expression levels had more severe disease complications. A study of silent cerebral infarcts in children with SCD has shown a small but statistically significant increase in plasma-soluble L-selectin levels. However, another study of L-selectin gene polymorphisms and L-selectin levels showed no relationship among gene polymorphisms, L-selectin levels, and SCD complications.
Hydroxyurea (HU) has been postulated to exert its effects partially through its ability to decrease neutrophilia in SCD, and several studies have attempted to determine if it also has an effect on neutrophil activation and concomitantly on neutrophil expression of L-selectin. Saleh and colleagues found that institution of HU therapy had no effect on sL-selectin levels. However, Benkerrou and colleagues have reported that HU corrected the dysregulated expression of L-selectin, which they found otherwise to be decreased without exogenous activation, and that HU also increased neutrophil production of H 2 O 2 after stimulation, which had been decreased in SCD neutrophils from subjects not exposed to HU.
When hypoxia/reperfusion injury is produced in sickle mice, an exaggerated inflammatory response is elicited. Neutrophils also seem to have an important direct role in vasoocclusion, as shown by the studies of Turhan and colleagues. They demonstrated that SS RBCs bind readily to leukocytes when the latter are adherent to endothelial cells in sickle mice. However, the role of L-selectin, if any, in this process is unclear. When sickle mice were deficient in both P- and E-selectins, they had many fewer leukocytes adhering to the vascular walls and were protected from vasoocclusion precipitated by the proinflammatory cytokine tumor necrosis factor α (TNFα), as further discussed later. However, these and other in vivo studies did not report investigation of the role of L-selectin.
E-Selectin in Sickle Cell Disease
Endothelial P-selectin has been shown to bind circulating SS RBCs and contribute to vasoocclusion, whereas E-selectin seems to have a critical role to play in vasoocclusion by mediating rolling and initial adhesion of leukocytes to endothelium. Once adherent, leukocytes then “capture” SS RBCs, causing a growing blockade of small vessels ( Fig. 3 ).
Zennadi and colleagues have explored the relationship between the abnormal SS RBC and leukocyte activation. They have shown that, in vitro, coincubation of SS RBCs and mononuclear leukocytes (a mixture of lymphocytes and monocytes) activates the leukocytes, which then show increased adhesion to nonactivated cultured endothelial cells. Significant inhibition of leukocyte adhesion to endothelium was observed when the cultured human umbilical vein endothelial cells used in these studies were preincubated with monoclonal antibodies reactive with E-selectin. However, antibodies to P-selectin did not inhibit leukocyte adhesion in this model. Zennadi and colleagues also showed that the SS RBC adhesion receptors critical for this stimulation of leukocyte adhesion seem to be ICAM-4 (LW protein) and CD44. More recently, this group has shown that contact with SS RBCs can also induce neutrophil adhesion.
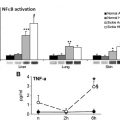
Blood cells from patients with SCD can also directly activate endothelial cells, causing upregulation of expression of VCAM-1, E-selectin, and ICAM-1. In studies by Brown and colleagues, this effect seemed to depend on TNFα and interleukin (IL)-1 produced by leukocytes. In an earlier and in some respects complementary study, Sultana and colleagues demonstrated the effect of SS RBCs on endothelial cells. They showed that interaction of SS RBCs with cultured human umbilical vein endothelial cells resulted in markedly increased indicators of cellular oxidant stress; interaction with normal RBCs did not have a similar effect. In addition, endothelial cell expression of several adhesion receptors, including E-selectin, were upregulated when endothelial cells were exposed to SS RBCs, and transendothelial migration of monocytes was also increased. Shiu and colleagues also showed that perfusion of cultured endothelial cells with SS RBCs caused increased gene expression of ICAM-1 and a more modest increase in VCAM-1 expression.
Mononuclear leukocytes from patients with SCD have also been described as having the capability of activating endothelial cells, although cell-cell contact was not required. Belcher and colleagues showed that SCD monocytes, but not normal monocytes, caused TNFα- and IL-1β-dependent activation of endothelial cells, which then led to nuclear translocation of nuclear factor-kB. However, Safaya and colleagues were unable to demonstrate that isolated monocytes from patients with SCD upregulated endothelial cell expression of L-selectin, E-selectin, VCAM-1, and ICAM-1 more than did normal monocytes.
In Vivo Studies in Animal Models of Sickle Cell Disease
In studies of the cremasteric vasculature of sickle mice, Hidalgo and colleagues in 2009 showed that inactivation of E-selectin led to protection from vasoocclusion in sickle mice, as well as protection from transfusion-related acute lung injury, also in a mouse model. However, in at least one similar model, only a P-selectin antibody, and not an E-selectin antibody, blocked the exaggerated hypoxia/reperfusion inflammatory response of sickle mice and enhanced blood flow in the affected vessels.
Additional studies were performed by Chang and colleagues, in which they studied the contribution of E-selectin as well as the activity of a small molecule selectin inhibitor, GMI-1070. GMI-1070 is a small carbohydrate molecule designed to mimic the bioactive domain of sLe x and present a conformation suitable for binding to the carbohydrate-binding domain of E-selectin, as analyzed by nuclear magnetic resonance techniques. The investigators first used enzyme-linked immunosorbent assays in which selectin chimeras were bound to the wells of the plates. They demonstrated that GMI-1070 bound to all 3 types of selectins, although its ability to bind E-selectin was about 100-fold higher than its ability to bind to L- and P-selectins in that assay. The IC 50 for E-selectin was 4.3 μM, whereas it was 423 μM and 337 μM for P-selectin and L-selectin, respectively.
They then used a flow chamber assay, in which human umbilical vein endothelial cells were cultured with TNFα to induce E-selectin expression or IL-4 and histamine to induce P-selectin expression. They found that adhesion of neutrophils was inhibited more readily by GMI-1070 when E-selectin expression was induced by TNFα than when P-selectin expression was induced by IL-4 and histamine.
The same authors then studied the activity of GMI-1070 in a mouse model of vasoocclusion. They treated mice with SCD that had undergone transplantation (C57BL/6 male mice, lethally irradiated and transplanted with Berkeley sickle cell mouse nucleated bone marrow cells), with both intraperitoneal injection of TNFα to induce cell adhesion and vasoocclusion and antibodies against P- and E-selectins (combined), GMI-1070, or control. Intravital microscopy of cremasteric muscle vessels was used to observe and measure cell adhesion and blood flow. They compared the ability of GMI-1070 and anti-P- and anti-E-selectin antibodies to affect leukocyte rolling and adhesion, as well as to abrogate vasoocclusion. Although they found that GMI-1070 slightly increased the percentage of rolling leukocytes, it also increased leukocyte rolling velocity and decreased the percentage of adherent leukocytes. The combined use of anti-P- and anti-E-selectin antibodies was even more effective in reducing leukocyte adhesion. GMI-1070 reduced the capture of SS RBCs by adherent leukocytes and improved blood flow in this model. The improvement in blood flow was equivalent when the effects of GMI-1070 and the combination of anti-P- and anti-E-selectin antibodies were compared. In addition, the investigators also studied the effect of GMI-1070 administered nearly 2 hours after the inflammatory stimulus and after the vasoocclusive process was established. In that setting, GMI-1070 also greatly increased blood flow and increased mouse survival. These studies confirmed the importance of both P- and E-selectins in vasoocclusion and the therapeutic potential of a compound such as GMI-1070, which could reduce E-selectin-mediated adhesion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree