CELL BIOLOGY OF BONE
Part of “CHAPTER 50 – PHYSIOLOGY OF BONE“
The lineages of bone-forming cells (osteoblasts) and bone-resorbing cells (osteoclasts) probably become separate early in development. Their function is controlled by a complex system of intercellular signals that involve not only systemic calcium-regulating and growth-regulating hormones but also local factors. Bone disease occurs when an imbalance exists between the functions of forming and resorbing cells. Thus, accelerated resorption and diminished formation exacerbate decreases in bone mass in osteoporosis. Excessive bone mass can occur because bone resorption is impaired, as in congenital osteopetrosis, or because formation is excessive and disorderly, as in virally induced avian osteopetrosis and Paget disease.
OSTEOBLASTS
The osteoblast, a highly specialized bone matrix–synthesizing cell, is derived from precursor cells in the periosteum or the stroma of the bone marrow called determined osteoprogenitor cells.10 Bone can also form in ectopic sites from undifferentiated mesenchymal cells in response to certain inducing agents, particularly demineralized bone matrix. This may be due to the effects of specific proteins produced by bone cells and deposited in matrix.11 Under these conditions, the sequence of endochondral bone formation is recapitulated; that is, cartilage is formed, mineralized, and then replaced by bone.
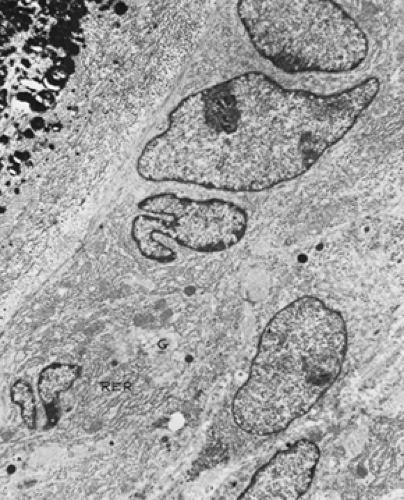
The mature osteoblast is a plump polygonal cell that has an eccentric nucleus, a prominent Golgi apparatus, and abundant rough endoplasmic reticulum (Fig. 50-5). These cells deposit a collagenous matrix and extend cytoplasmic processes into this matrix. As they complete their synthetic activity, they become buried in their own matrix and are called osteocytes. Osteoblasts may also stop producing matrix but remain on the bone surface; these lining cells or resting osteoblasts can be the sites for new remodeling cycles of activation, resorption, and formation. Lining cells may also be reactivated to functional osteoblasts in response to mechanical loading.
Osteoblasts produce most of the constituents of bone matrix; however, many proteins are taken up by matrix from the circulation, including α2-HS-glycoprotein and albumin.12 Osteoblasts produce alkaline phosphatase and release it systemically. Hence,
serum alkaline phosphatase activity correlates with osteoblastic activity. BGP is also released into the circulation from osteoblasts and provides another marker of their activity.13 When procollagen is converted to collagen, large N- and C-terminal peptides are released. These can be measured in the circulation and reflect the overall rate of collagen synthesis in the body.
serum alkaline phosphatase activity correlates with osteoblastic activity. BGP is also released into the circulation from osteoblasts and provides another marker of their activity.13 When procollagen is converted to collagen, large N- and C-terminal peptides are released. These can be measured in the circulation and reflect the overall rate of collagen synthesis in the body.
Osteoblasts have receptors for PTH and 1,25-dihydroxyvitamin D3 [1,25(OH)2 D3] and for systemic growth regulators.14 They apparently are the major source of the autocrine factors that regulate local bone turnover, including cytokines, prostaglandins, and bone-derived growth factors. Osteoblasts may trigger bone resorption in the remodeling process. This activation step may involve shape changes or secretion of collagenase and related metalloproteinases as well as other proteolytic enzymes, such as plasminogen activator.15 These changes may enhance the access of osteoclasts to the mineralized bone surface.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree