CD1d-Restricted Natural Killer T Cells
Albert Bendelac
INTRODUCTION AND DEFINITION
Natural killer T (NKT) cells are cluster of differentiation (CD)1d-restricted T cells that use semi-invariant αβ T-cell receptors (TCRs) and reside as long-lived effector cells in lymphoid tissues and in the microvasculature of organs such as the lung and the liver. They promptly release a broad range of cytokines and chemokines in response to microbial and self-lipid antigens presented by CD1d glycoproteins, exerting a protective or pathogenic role in infection, inflammation, allergy, and cancer. The NKT lineage is best defined by expression of Zbtb16, which encodes promyelocytic leukemia zinc factor (PLZF), a master transcription factor that directs acquisition of innate-like effector properties during thymic development.
The NKT lineage is a member of the larger family of so-called innate-like lymphocytes, which includes B1 B cells, marginal zone B cells, intraepithelial γδ T-cell sublineages, the CD8αα TCRαβ population of intestinal lymphocytes, and the MR1-restricted mucosal associated invariant T cells.1 Like NKT cells, these lineages express semi-invariant TCRs or B-cell receptors encoding specificity for conserved microbial and self-ligands. Their “canonical” antigen receptors are sufficient to instruct lineage differentiation during development in the thymus or bone marrow, in a process matching antigen specificity with specialized effector functions and homing to dedicated tissue environments. These stereotypical properties, which are reminiscent of truly innate lymphoid cells, such as natural killer (NK) cells, represent a distinct host defense strategy, perhaps corresponding to an early phase of evolution of adaptive T- and B-cell immunity.
CANONICAL NATURAL KILLER T T-CELL RECEPTORS
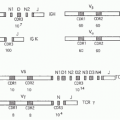
In mice, the vast majority of CD1d-restricted NKT cells express the semi-invariant Vα14-Jα18 TCRα chain paired with β chains made of Vβ8, Vβ7, or Vβ2 joined to variable DβJβ segments, whereas the homologous Vα24-Jα18 chain associated with Vβ11 is used in humans2 (Table 18.1). The “canonical” sequence of the TCR alpha chain is entirely encoded in the genome, although alterations due to nucleotide trimming and N additions can be tolerated if they preserve the antigenic specificity of the TCR. These canonical rearrangements arise randomly and at very low frequency in both fetal and adult life, but massive thymic expansion post-TCR expression ensures a high frequency of NKT cells among recent thymic emigrants.3
Other semi-invariant TCRs have been identified among mouse CD1d-restricted NKT cells, including Vα10-Jα50/Vβ8,4 Vα3.2-Jα9/Vβ8, and Vα8/Vβ8,5 but their combined frequency is modest compared to the dominant Vα14-Jα18 TCR. Nevertheless, these cells appear to adopt a similar NKT effector phenotype, likely because they follow the same thymic developmental pathway.
ANTIGENIC LIGANDS OF NATURAL KILLER T CELLS
Cluster of Differentiation 1d
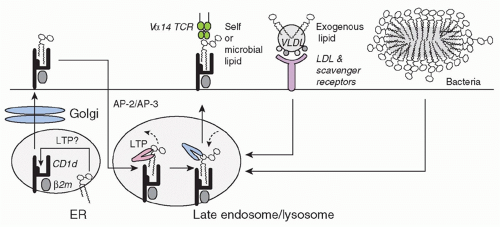
CD1d is one of five members of the mammalian family of lipid-presenting, β2-microglobulin-associated, major histocompatibility complex (MHC)-like CD1 molecules, and the only one conserved in mouse.6,7 The mouse CD1 locus contains cd1d1 encoding a functional surface glycoprotein and a duplicated gene cd1d2, which is generally poorly expressed and, in the C57 background, is inactivated by a frameshift mutation preventing surface expression.8 CD1d is found constitutively on the cell surface of most antigen-presenting cells, including dendritic cells (DCs), macrophages, and B cells, with particularly high levels on marginal zone B cells.9 Of relevance to NKT-cell development, CD1d is also prominently but transiently displayed on cortical thymocytes. It is also expressed by endothelial cells and hepatocytes.
CD1d is assembled in the endoplasmic reticulum by association with β2-microglobulin before reaching the cell surface and undergoing extensive rounds of internalization and recycling between the late endosome/lysosome and the plasma membrane10,11,12 (Fig. 18.1). The rapid rate of internalization depends upon a tyrosine motif encoded in its cytoplasmic tail, which binds adaptor protein (AP)-2 and AP-3 in mouse, and AP-2 in human. This intense recycling accounts for the steady state accumulation of CD1d in the late endosome/lysosome for mouse or late endosome for human. While a diversity of exogenous and endogenous lipids can load CD1d in various compartments,13,14,15 the late endosome/lysosome environment is optimized for lipid antigen acquisition due to the presence of efficient glycolipid processing enzymes and lipid transfer proteins such as saposins,16,17,18 Gm2 activator,16 Niemann-Pick type C2 protein,19 and, in humans, CD1e.20 In this acidic compartment, short or polyunsaturated lipids loaded at the cell surface are quickly removed and replaced by long and saturated lipids that bind more stably to CD1d but require lysosomal transfer proteins for loading.21 Unlike MHC class II, CD1d expression and its presentation of lipid antigens at the cell surface are
independent of toll-like receptor (TLR) signaling and DC maturation.22 Thus, immature DCs can readily present purified lipid antigens to NKT cells and become activated upon CD40 engagement by NKT cells.
independent of toll-like receptor (TLR) signaling and DC maturation.22 Thus, immature DCs can readily present purified lipid antigens to NKT cells and become activated upon CD40 engagement by NKT cells.
TABLE 18.1 Semi-invariant T-Cell Receptors of Cluster of Differentiation 1d-Restricted Natural Killer T Cells | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Lipid Ligands of Natural Killer T cells
bial α-glycosylated Lipid Ligands
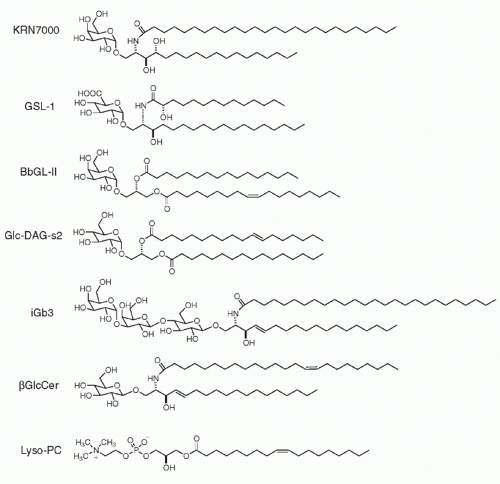
The first universal NKT ligand emerged from studies of marine sponge extracts that prolonged survival of mice bearing B16 melanoma.23 The active principle of the sponge Agelas mauritianus was an alpha-branched galactosylceramide, which after slight modification led to the synthesis of an extremely potent variant named KRN7000, commonly referred to as αGalCer24,25 (Fig. 18.2). Over 95% of mouse and human NKT cells recognize αGalCer, irrespective of their variable CDR3 β sequence.26,27 Furthermore, the mouse CD1d-αGalCer tetramers stain the NKT cells of both human and nonhuman primates,28,29 attesting to the high degree of conservation of this recognition system.
The marine sponge lipid turned out to be closely related to microbial lipids found in some gram-negative lipopolysaccharide (LPS)-negative bacteria. Notably, Sphingomonas, a member of the class of α-proteobacteria and a ubiquitous bacterium found in terrestrial and marine environments (including as a bacterial symbiont of sponges), uses alphabranched glycuronylceramides as a substitute for LPS in the outer membrane of its cell wall.30 It can infect dendritic cells and macrophages to activate NKT cells.31,32 Other structurally related α-glycosylated lipids, such as α-galactosyldiacylglycerol, found in Borrelia burgdorferi, the agent of Lyme disease,33 and α-glucosyldiacylglycerol, found in Streptococcus pneumoniae,34 also activate mouse and human NKT cells. Such α-branched glycolipids have not been reported in vertebrates, suggesting that they represent a pathogen signature that the canonical NKT TCR may have evolved to recognize.
Endogenous β-glycosylated Lipid Ligands
Vα14 NKT cells are spontaneously autoreactive to CD1dexpressing cells.35 For example, NKT hybridomas or clones
can be activated to secrete modest amounts of cytokines when cultured in the presence of fresh thymocytes, DCs, and various tumor cell lines.8,35,36,37,38 This autoreactivity and the agonist ligands involved appear to be central not only for NKT-cell thymic development,36 but also for their response to various microbial infections, especially when the pathogens themselves do not express NKT ligands.31,39 The identification of self-ligands is therefore of major relevance to key aspects of the biology of NKT cells.
can be activated to secrete modest amounts of cytokines when cultured in the presence of fresh thymocytes, DCs, and various tumor cell lines.8,35,36,37,38 This autoreactivity and the agonist ligands involved appear to be central not only for NKT-cell thymic development,36 but also for their response to various microbial infections, especially when the pathogens themselves do not express NKT ligands.31,39 The identification of self-ligands is therefore of major relevance to key aspects of the biology of NKT cells.
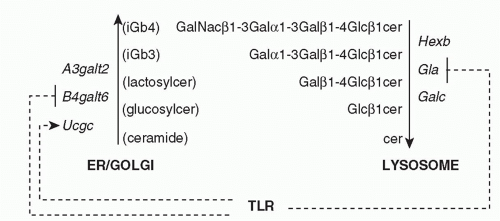
In the mouse system, recognition of self-ligands by Vα14 NKT cells is largely dependent upon CD1d endosomal trafficking,40,41 suggesting the recognition of endosomal/lysosomal lipid antigens. This may not be the case for their human counterpart, Vα24 NKT cells.42 The search for a lysosomally-loaded self-ligand recognized by NKT cells led to the identification of a trihexosylceramide, iGb3, which is synthesized in the Golgi as an intermediate in the biosynthetic pathway of iGb4 and is also generated in the lysosome after degradation of iGb4 by β-hexosaminidase AB.43 iGb3 is further degraded into LacCer by α-galactosidase A (Fig. 18.3). Mice lacking β-hexosaminidase B lacked 95% of their NKT cells, a defect associated with the impaired ability of their thymocytes to stimulate autoreactive NKT hybridomas. Conversely, unlike αGalCer, iGb3 is recognized with a Vβ7 > Vβ8 > Vβ2 hierarchy of affinities that mirrors the relative selection of NKT thymic precursors.44 Furthermore, DCs lacking α-galactosidase A exhibited greater spontaneous stimulation of NKT cells,45 consistent with the demonstrated accumulation of iGb3.46,47,48
Among several genes encoding Galα1-3Gal transferases, a3galt2 is considered more specialized for iGb3 synthesis. As a3galt2 null mutant mice did not show NKT cell defects49 and the human homologous gene appeared dysfunctional,50 iGb3 may not be the sole endogenous glycosylceramide recognized by mouse and human NKT cells. Other reports have suggested that β-galactosylsylceramide51 and β-glucosylceramide52 might also function as natural ligands of NKT cells, but these claims have not yet been widely replicated. While the respective importance of all these candidate ligands remains to be further studied in physiological and pathological conditions, it is possible that NKT cells might recognize multiple endogenous β-glycosylceramides as weak agonists.
Importantly, several enzymes involved in the synthesis and degradation of β-glycosylceramides appear to be coordinately regulated in conditions associated with NKT-cell activation45,52,53 (see Fig. 18.3). For example, TLR signaling was shown to specifically downregulate α-galactosidase A in the degradation pathway,45 leading to increased iGb3.46 In the biosynthetic pathway, TLR signaling upregulated β-glucosylceramide synthase while downregulating lactosylceramide synthase, thus increasing β-glucosylceramide.52 Collectively, these findings suggest that microbial organisms lacking NKT ligands can nevertheless activate NKT cells through TLR-mediated accumulation of endogenous glycosphingolipid ligands.
Structural Basis of Lipid Recognition by Natural Killer T cells
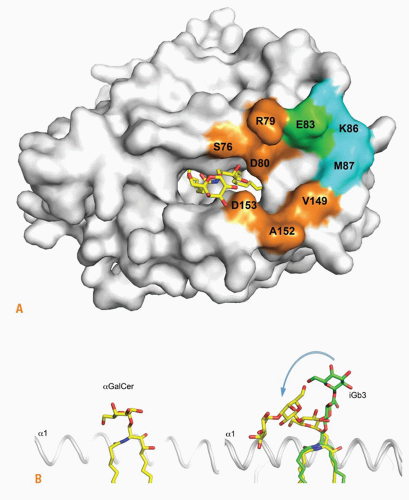
The lipid-binding pocket of CD1d is particularly well adapted to bind self- and microbial glycosphingolipids, with the acyl chain in the A’ hydrophobic pocket and the sphingosine chain in the F’ hydrophobic channel.56,57,58 The hydroxyl groups of the sphingosine emerge from the groove to establish hydrogen bonds with the α1 helix Arg79 and Asp80, while the galactose is stabilized though hydrogen bonds between its 2- and 3-hydroxyl groups and Asp153 of the α2 helix. Thus, the protruding sugar is solidly anchored in a position parallel to the plane of the α-helices, explaining the exquisite stimulatory properties of several carbohydrate hydroxyl groups. In contrast, the trisaccharide chain of iGb3 protrudes in orthogonal orientation to the plane of the α-helices, with the proximal glucose forming hydrogen bonds with the α2 helix, in particular Asp153 and Thr156, whereas the position of the ceramide backbone is similar to αGalCer (Fig. 18.4).
How could such highly dissimilar structures be recognized by the same TCR? In the ternary structure of the human TCR-CD1d-αGalCer complex, the TCR docked parallel to and at the extreme end (F’ pocket) of the CD1d binding groove, enabling a lock-and-key type of interaction with the solidly anchored, rigid αGalCer antigen. The docking was parallel to the long axis of the binding groove, contrasting with the diagonal footprints observed for MHC-restricted TCRs.59 The CDR3α loop encoded by the conserved germline Jα18 segment provided a majority of contacts, straddling the antigen binding cleft and engaging electrostatic interactions with α-GalCer, the α1 and the α2 helices of CD1d. CDR1α interacted solely with αGalCer, and CDR2β formed a stretch of interactions with the α1 helix. Consistent with the great diversity of CDR3β usage, this loop contact was limited to a single van der Waals contact with Gln150 of the α2 helix.
The ternary structure of the TCR-CD1d-iGb3 complex was recently elucidated. Unsurprisingly, given the conservation of Jα18 and CD1d in both structures, the overall footprint of the TCR on the CD1d-glycolipid surface was unchanged compared with CD1d-αGalCer. Strikingly, however, the TCR appeared to simply “squash” the trisaccharide headgroup of iGb3 over the α2 helix of CD1d, with the proximal β-linked glucose molded into an orientation similar to the α-linked galactose of αGal-Cer (see Fig. 18.4). Each of the three sugars made stabilizing polar and van der Waals interactions with CD1d residues. The proximal and second sugars also contacted the TCR CDR2α loop, which is not involved in CD1d-αGalCer recognition. The importance of the distal sugar is reflected by the lack of detectable stimulation by lactosylceramide where the third sugar is absent. These conformational changes imply a highly dynamic interaction process during the association phase. The energy penalty incurred for binding self-ligands could be overcome in specific conditions that strengthen the immune synapse. For example, this may occur during TLR-induced inflammation, which upregulates lymphocyte function-associated antigen (LFA-1) and intercellular adhesion molecule (ICAM-1) integrin interactions, or during thymic development, which involves homophilic engagement of signaling lymphocytic activation molecule (SLAM) family receptors.
Additional crystallographic studies further illustrated the difference between self- and foreign-antigen recognition. The α-glycosyldiacylglycerol antigens of Borrelia burgdorferi33,60,61 and Streptococcus pneumoniae34 adopted configurations relatively similar to αGalCer, although some induced fit was observed for both CD1d and the glycolipid upon TCR binding. In the case of Streptococcus pneumoniae, an unusual sn2 alkyl chain, vaccenic acid (with an unsaturation at C7), was important for favorable positioning of the glucose. After insertion of a stretch of hydrophobic aminoacids in the CDR3β loop in order to enhance binding to CD1d, other self-lipids such as β-GalCer, Galα1-4GlcCer (lactosylceramide), and phosphatidylinositol could be recognized.62 Although somewhat contrived, this system revealed a similar bending of the β-linkage between the proximal sugar and the ceramide backbone as seen for iGb3, raising the possibility that several β-linked self glycolipids might serve as autoantigens.
DEVELOPMENT AND HOMEOSTASIS OF NATURAL KILLER T CELLS
Development
The major determinant of NKT cell development is the semi-invariant TCR, which, upon binding to CD1d ligands expressed by cortical thymocytes, provides the signals required for induction of the lineage-specific transcription factor PLZF. Therefore, insights into the peculiar nature and context of TCR engagement and its downstream signaling have considerably advanced our understanding of the molecular mechanisms of NKT-cell development.
T-Cell Receptor Expression and Positive Selection
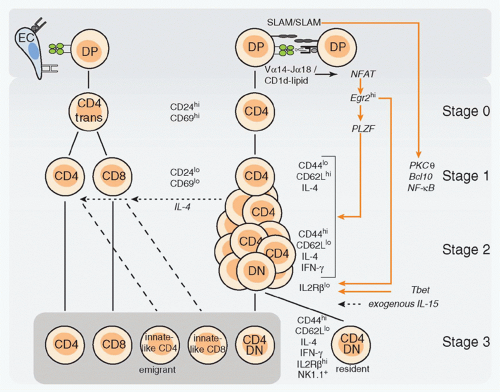
The use of CD1d-αGalCer tetramers specific for the canonical mVα14-Jα18/hVα24-Jα18 TCRs has revealed a sequence of selection, expansion, and differentiation events preceding the terminally differentiated NK1.1+ stage.3,63,64 As illustrated in Figure 18.5, NKT cells originate from mainstream thymocyte precursors that transit through the pre-TCRα+TCR+ stage to reach the CD4+CD8+ double positive (DP) stage, where stochastic Vα-Jα rearrangements lead to expression of canonical Vα14-Jα18/Vβ8, Vβ7 or Vβ2 TCRs. These rearrangements involve distal gene segments that require prolonged cell survival mediated by Bcl-xL, whose expression depends on HEB-induced RORγt.65,66,67 All Vβ families can pair with the Vα14-Jα18 chain, but only the biased set of Vβs confers specificity for the endogenous ligands. Although Vβ8 predominates among mature NKT cells, the most enriched Vβ family relative to preselection frequency is Vβ7, followed by Vβ8 and Vβ2.44 This is consistent with the observation that endogenous ligands displayed on the surface of thymocytes activate the Vα14-Jα18 TCRs with a Vβ7 > Vβ8 > Vβ2 hierarchy of affinity, and that in mice expressing very low levels of CD1d, NKT cells showed increased representation of Vβ7.44,68,69,70,71 Notably, this hierarchy of affinity parallels that of iGb3 but is different from αGalCer, which favors Vβ8. Thus, the naturally selected Vβ repertoire seems to precisely reflect the affinities of the preselected repertoire for endogenous ligands, suggesting that negative selection plays little role in shaping the Vβ repertoire. However, this does not imply that NKT cells are impervious to negative selection because transgenic overexpression of CD1d decreased the relative frequency of the high affinity Vβ7+ cells and exposure to αGalCer-induced deletion of NKT thymocytes.69
Developmental Stages
The earliest signaled NKT precursors are rare CD4+CD8+ DP cells that express the semi-invariant TCR. These cells become CD4+ single positive (SP) cells expressing high levels of CD24, a marker of immature cortical thymocytes, as well as CD69, a marker of positive selection.64 This is the socalled stage 0 of NKT development. As CD24highCD4+ cells mature into the next CD24lowCD4+ stage 1, they undergo several rounds of cell division.3 Cell cycle may be initiated as early as stage 0, as suggested from the analysis of cyclin D2-or c-Myc-deficient mice.72,73 The lineage expansion following positive selection ensures the high frequency that is critical for innate immunity. During this phase, a fraction of CD4+
cells downregulates CD4 to become CD4-CD8- double negative (DN) T cells,64 acquiring a more restricted Th1 cytokine and chemokine profile and an enhanced ability to reject tumors.74,75,76 Dividing cells first activate their interleukin (IL)-4 locus (stage 1) then interferon (IFN)-γ (independently of both stat6 and stat4) as they upregulate CD44 and downregulate CD62L (stage 2).3,63,77 Thus, unlike other αβT cells, postselection NKT lineage cells undergo a sequence of events that is reminiscent of the antigen-driven activation, expansion, and effector differentiation of mature T cells.78
cells downregulates CD4 to become CD4-CD8- double negative (DN) T cells,64 acquiring a more restricted Th1 cytokine and chemokine profile and an enhanced ability to reject tumors.74,75,76 Dividing cells first activate their interleukin (IL)-4 locus (stage 1) then interferon (IFN)-γ (independently of both stat6 and stat4) as they upregulate CD44 and downregulate CD62L (stage 2).3,63,77 Thus, unlike other αβT cells, postselection NKT lineage cells undergo a sequence of events that is reminiscent of the antigen-driven activation, expansion, and effector differentiation of mature T cells.78
NKT lineage cells emigrate from the thymus after upregulation of the S1P1 receptor,79 as dividing effector-type cells. They represent up to 5% of recent thymic emigrants in the mouse spleen.3,80 Within a couple of days after emigration, they express NK lineage receptors, including the activating NK1.1 and NKG2D receptors and the inhibitory CD94/NKG2A, Ly49A, C/I, and G2 receptors. Acquisition of this terminal differentiation program is associated with cessation of division and with upregulation of IL2Rβ, which is necessary for IL-15 signaling. Intriguingly, a fraction of NKT thymocytes downregulate the S1P1R and remain as permanent residents in the thymic medulla, where they undergo the same terminal maturation program.79 These thymic residents appear to be absent in humans.81
Recent thymic NKT emigrants and their immediate precursors, stage 2 NKT thymocytes, express neuropilin 1 (Nrp-1), a transmembrane receptor for vascular endothelium growth factor and semaphorin family members.82 Nrp-1 represents a convenient marker of recent thymic NKT emigrants, as it remains expressed for a few days and is downregulated after terminal maturation to stage 3.
Homotypic Thymocyte-Thymocyte Interactions
The thymic cell types involved in presenting NKT ligands have been thoroughly investigated. Unlike MHC class I or class II, CD1d is prominently expressed on cortical thymocytes, which is consistent with the ability of cortical thymocytes to stimulate NKT hybridomas.36 Bone marrow chimera experiments demonstrated that NKT-cell development required CD1d expression by cortical thymocytes but not radioresistant stromal cells.83,84,85 This is radically different from conventional T-cell development that is driven by MHC expression on thymic epithelial cells. Transgenic experiments using promoters for Lck, MHC class I, or MHC class II to redirect CD1d to various cell compartments in CD1d-deficient hosts suggested that expression on cortical thymocytes was necessary and sufficient for lineage development.70,86,87,88 This conclusion was recently confirmed after
conditional deletion of CD1d using Cd4-Cre.89 Mixed chimera experiments using CD1d knockout pLck-CD1d transgenic TCR Cα knockout bone marrow (where thymocyte development is arrested at the DP stage) as the sole source of CD1d expression demonstrated that expression of CD1d solely on cortical thymocytes was sufficient for the full differentiation of NKT cells.87 Intriguingly, however, the transition to the NK1.1 positive stage was partially impaired after emigration to peripheral tissues, although it was preserved for the cells remaining as residents in the thymus. Terminal differentiation was fully restored if CD1d was re-expressed on MHC class II expressing cells or if the cells were transferred into wild-type recipients.80,87
conditional deletion of CD1d using Cd4-Cre.89 Mixed chimera experiments using CD1d knockout pLck-CD1d transgenic TCR Cα knockout bone marrow (where thymocyte development is arrested at the DP stage) as the sole source of CD1d expression demonstrated that expression of CD1d solely on cortical thymocytes was sufficient for the full differentiation of NKT cells.87 Intriguingly, however, the transition to the NK1.1 positive stage was partially impaired after emigration to peripheral tissues, although it was preserved for the cells remaining as residents in the thymus. Terminal differentiation was fully restored if CD1d was re-expressed on MHC class II expressing cells or if the cells were transferred into wild-type recipients.80,87
Homophilic Signaling Lymphocytic Activation Molecule-f Family Interactions
A major pathway that is selectively recruited by the thymocyte-thymocyte interactions involves homophilic binding of SLAM family receptors, mainly Slamf1 (SLAM) and Slamf6 (Ly108), which are expressed by cortical thymocytes, but not by thymic epithelial cells.90 These partially redundant receptors signal through the adaptor SLAM-associated protein (SAP) and the kinase Fyn, explaining earlier reports of developmental arrest in SAP- or Fyn-deficient NKT precursors.91,92,93,94,95 SAP- and Fyn-deficient NKT thymocytes were blocked at stage 0 and could not be rescued by expression of Bcl-2 or Bcl-xL. They showed lower induction of CD69,90 suggesting defective signaling by the TCR, perhaps due to a role of Slamf-SAP-Fyn signaling in stabilizing the immune synapse.96 Individual ablation of Slamf1 or Slamf6 resulted in a modest twofold defect in the expansion of the NKT lineage between stages 1 and 2. However, in “pseudo-double mutant” mixed chimeras where Slamf1/CD1d double deficient NKT precursors were forced to see their ligands on Slamf6-deficient thymocytes, a > 10-fold reduction of NKT cells was observed, demonstrating a requirement of Slamf receptors at the time of TCR engagement by CD1d ligands.90 Notably, the Slamf locus exhibits considerable polymorphism, which may underlie some of the reported variations in NKT-cell frequencies in different mouse strains and in humans. Genetic studies have provided support for this hypothesis by identifying the lack of expression of Slamf1 by cortical thymocytes in the nonobese diabetic (NOD) strain, which is spontaneously NKT deficient.97
Signaling in the Natural Killer T-Cell Microenvironment
Engagement of the semi-invariant TCR activates the same Ras/MAP kinase and calcineurin pathways as reported for MHC-restricted TCRs during positive selection.98,99 Notably, NKT thymocytes show elevated and sustained expression of the early growth response (Egr) factors 1 and 2 compared with the weak and transient expression observed in MHCrestricted thymocytes.100 Egr1 is thought to be downstream of the Ras/MAP kinase pathway, whereas Egr2 is mainly induced by the calcineurin/nuclear factor of activated T cells (NFAT) pathway. Egr1 and Egr2 mediate the survival of MHC-restricted T-cell precursors after positive selection through induction of Bcl2 and Bcl-xL.101,102,103 In the case of NKT cells, however, sustained Egr elevation has specific lineage-determining consequences. Egr2 directly binds to the promoters of NKT lineage-specific genes such as Zbtb16, encoding PLZF, and Il2rb, encoding the β chain of the IL-15 receptor, and it is required for their induction. This suggests a direct connection between the peculiar signaling emanating from the TCR synapse and these NKT lineage checkpoints.100 The sustained elevated Egr levels likely result from the TCR recognition of agonist ligands36,43 or from the Slamf-SAP-mediated stabilization of the immune synapse.96 As some Zbtb16 messenger ribonucleic acid induction was detected in SAP-deficient NKT precursors, SAP may not be absolutely required for PLZF induction.104
TCR and Slamf-SAP-Fyn signaling both involve the canonical NF-κB pathway through PKCθ and Bcl-10.105,106 Mice lacking these downstream signaling components showed partial defects in NKT-cell development,107,108 as further detailed in the following.
Thus, NKT-cell development is tightly dependent on the signaling elicited through the TCR and Slamf-SAP-Fyn pathways, the specific contributions of which remain to be dissected. Notably, redirecting the expression of MHC class II proteins on cortical thymocytes through ectopic expression of the transcription factor CIITA led to the differentiation of “innate-like” CD4+ thymocytes in a SAP-dependent manner. These cells resembled stage 2 NKT cells and expressed PLZF, reinforcing the notion that homotypic thymocyte-thymocyte interactions, Slamf-SAP-Fyn signaling, and PLZF define a dedicated thymic pathway for the production of innate-like effector T cells.109,110,111,112 In that context, the reciprocal expression patterns of CD1 and Slamf receptors by thymocytes and of MHC proteins by epithelial cells may well serve the primary purpose of creating different niches for different thymic lineages.90
Cluster of Differentiation 4 and Cluster of Differentiation 8 Coreceptor Expression
NKT cells originate from the same pool of DP precursors as MHC-restricted T cells and emerge from thymic selection as CD4 SP cells expressing the CD4 lineage factor ThPOK/c-Krox in a Gata-3-dependent manner.113,114 A fraction goes on to downregulate CD4 and acquire the DN phenotype, but they still stably express ThPOK, which is essential to downregulate CD8. In humans, some NKT cells can express CD8αα homodimers. ThPOK-deficient NKT cells did not express CD4, and some of them also failed to repress CD8, but otherwise they appeared to develop normally.113 Their functional properties, however, have not been fully assessed. CD8α-deficient mice showed a modest but significant bias toward the selection of high-affinity Vβ7 TCRs compared with littermate controls.83 A similar bias was observed after anti-CD8 antibody treatment, suggesting a minor role of CD8 as a coreceptor for CD1d. Transgenic expression of CD8α resulted in the disappearance of NKT thymocytes, suggesting a role in negative selection,83 a conclusion subsequently challenged when the transgenic model was found to have impaired Vα-Jα rearrangements.114
Thus, while NKT-cell development does not appear to rely on CD4 or CD8 coreceptors, the induction of ThPOK and CD4 in this lineage may simply reflect the path of high
affinity TCRs whose signaling is coreceptor independent.115 However, the subsequent CD4 downregulation in up to 50% to 70% of NKT cells, despite persistent ThPOK expression, remains to be explained.
affinity TCRs whose signaling is coreceptor independent.115 However, the subsequent CD4 downregulation in up to 50% to 70% of NKT cells, despite persistent ThPOK expression, remains to be explained.
Cluster of Differentiation 28-B7 Interactions
A modest decrease in the thymic expansion of NKT cells was reported in mice lacking CD28 or B7,116,117 but the nature of the B7-expressing cell type (epithelial cells or DCs) has not been determined.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree