Introduction
Ageing is a highly heterogeneous process and deterioration in organ function, including the heart, often does not correlate well with chronological age. A complex mix of influences combine to influence cardiovascular function in older people, including intrinsic ageing, the cumulative effect of multiple underlying diseases, and many environmental or external influences (such as frequency and intensity of exercise, cigarette smoking and diet). Separating the effects of intrinsic ageing from subclinical disease is challenging, and teasing out the additional contributions of environmental and lifestyle exposures even more problematic. Accepting these difficulties, it is still important to have a basic knowledge and understanding of the effects of intrinsic ageing on the heart to enable accurate interpretation of the effect of systemic disease on cardiovascular function in older people (see Figure 35.1). ‘Healthy’ or intrinsic cardiac ageing is associated with gradual decline in organ function and homeostatic reserve. The effects include reduced maximal aerobic exercise capacity and a decreased threshold for clinical expression of many age-related cardiac and non-cardiac diseases. However, healthy cardiac ageing by itself should not cause symptoms or limit usual activities of daily living.
Although there is no uniform ageing heart phenotype, some general principles are apparent, and we have attempted to summarize these. In this regard there are established and emergent data from multiple sources including in vitro work, animal models and clinical studies. This chapter aims firstly to summarize effects of ageing on cardiac structure, function and homeostatic reserve. The potential impact of these changes on the manifestation of various systemic (non-cardiac) diseases is then considered.
Changes in Cardiac Structure with Ageing
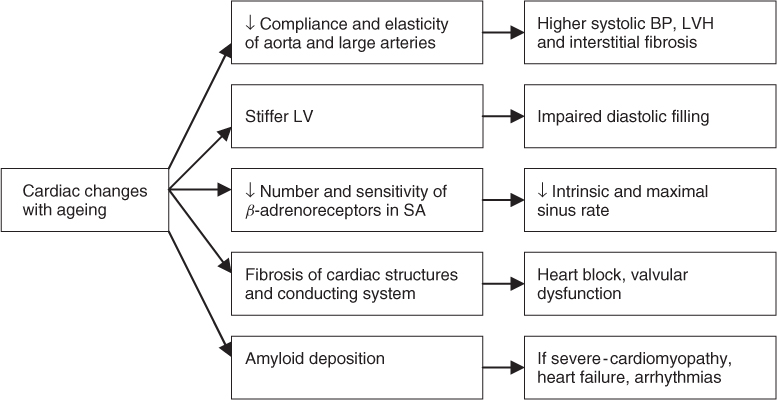
For most solid organs age-related atrophy is the norm; however, magnetic resonance imaging (MRI) studies of the ageing heart show approximately 10% increase in cardiac mass from the age of 20 to 80 years in both men and women;1 the ‘healthy’ older left ventricle is characterized by an increased mass-to-volume ratio.2 Autopsy series confirm a high prevalence of left ventricular hypertrophy (LVH) in elderly subjects.3–5 This hypertrophy is caused mainly by increased impedance to left ventricular ejection due to reduction in elasticity and compliance of the aorta and large arteries with resultant higher systolic arterial pressures; age-related degenerative aortic valve disease with sclerosis and thickening of valve leaflets and proximal bulging of the interventricular septum may also contribute; however, the contribution of these changes to LVH has not been definitively proven.
Data on cardiac changes in very elderly subjects are limited, with small numbers studied and often apparently contradictory results. Varying results are at least in part explained by differing rates of underlying disease, with coronary artery disease particularly common in males and hypertension in females.6 As a result, in unselected very elderly subjects, reduced left ventricular systolic function and wall motion abnormalities are over-represented in males while females are particularly likely to have ventricular hypertrophy, atrial dilatation and moderate-to-severe mitral and tricuspid valve dysfunction.
Studies in older populations comprehensively screened for cardiovascular disease, particularly hypertension and coronary artery disease, have generally shown that the morphological cardiac changes described above are less marked in disease-free older hearts.6 For example in the longitudinal Framingham cohort study, the association of LVH with increasing age was explained mainly by higher blood pressure, overweight, smoking and diabetes mellitus.7 These findings reinforce how difficult it is to distinguish between cardiac changes secondary to intrinsic ageing and changes as a result of disease or environmental exposures. It is likely that certain structural changes classically thought to be primarily manifestations of intrinsic ageing are in fact also influenced by lifestyle or environmental factors; reports of partial reversal of age-associated changes with interventions such as exercise lends further credence to this hypothesis.8, 9
Microscopic changes in the ageing heart are well described and include a decrease in number of myocytes with hypertrophy of remaining cells. Convincing mechanistic explanations for these changes are lacking although tissue hypoxia secondary to reduced capillary density has been postulated.10 Contrary to previous belief, adult myocardial cells remain capable of active division and this has been demonstrated in vitro in response to myocardial injury.11 There is an age-associated increase in other cardiac cell types and materials including interstitial fat, lipofuscin pigment and fibroblast numbers (and activity).11 The magnitude and functional importance of cardiac interstitial fibrosis is debated12 but age-related valvular fibrosis and calcification is common. The latter often causes valvular regurgitation or stenotic gradients, manifest on echocardiography or clinically as murmurs on auscultation. Previously thought to be benign, ‘senile aortic sclerosis’ is now recognized to be associated with significant increases in cardiovascular and total mortality.13
Intracellular deposits of amyloid are common in the older heart. Distinct patterns of atrial appendage amyloid and more generalized deposition are recognized.14 In post-mortem studies amyloid deposits have been found in the majority of aged hearts with the highest prevalence (80–100%) reported in Japanese populations.15 Although most elderly patients do not have symptoms from cardiac amyloid, some will develop extensive infiltration resulting in a clinical cardiomyopathy that can in turn be associated with heart failure and arrhythmias.15 The electrocardiograph (ECG) may show low voltage complexes and on echocardiography, the myocardium has a characteristic bright ‘speckling’ appearance with other features of a restrictive physiology. In advanced cases virtually every cardiac structure can be infiltrated. Age-related reduction in the number of functional pacemaker cells in the sinoatrial node and myocyte cells in specialized conducting tissues are well described and are clinically manifest by ECG changes and predisposition to dysrhythmias such as ‘sick sinus syndrome’.16
Biochemical changes with age have been reported including alterations in sarcoplasmic reticulum and Ca2+ uptake across membranes. In isolated senescent cardiac tissue slower myosin isoenzymes and decreased adenosine triphosphatase activity are also described.17 These biochemical alterations result in prolongation of isovolumetric relaxation. These changes may be of functional importance, particularly with regard to reduced diastolic function and cardiac failure with preserved ejection fraction.
Changes in Cardiac Physiology with Ageing
Healthy ageing is associated with little change in systolic function or cardiac output at rest. However, age-related changes in diastolic function are apparent on echocardiography or cardiac MRI.18 There are progressive changes in the pattern of left ventricular filling, with numerous indirect measures of diastolic function showing deterioration with advancing age; the early phase of diastolic ventricular filling is delayed and maximal flow rate across the mitral valve is reduced; as a result a much greater proportion of ventricular filling occurs late in diastole during atrial contraction. These changes in diastolic function seem to be largely independent of disease. However, their precise aetiology and in particular the role of reduced left ventricular compliance and myocardial relaxation patterns remains to be definitively established.2, 19 For most older people these changes are not of major consequence. However, the development of atrial fibrillation (AF) (and loss of the late ventricular filling phase) has much more severe adverse consequences in the older heart, with greatly reduced cardiac homeostatic reserve and maximum aerobic exercise capacity.
There is an age-related reduction in cardiac responsiveness to adrenergic stimuli, resulting in a decrease in both intrinsic and maximal sinus heart rate.20 During exercise or other stressful stimuli, the increase in heart rate is attenuated in older people; at any given submaximal work-rate the heart rate rise is reduced and the older heart relies on dilatation and increased stroke volume to increase cardiac output (working on the physiological principles of the Starling curve). This compensatory response is effective at low or moderate workloads; however, cardiac reserve is reduced, with a reduction in maximal cardiac output in the older heart. These changes appear to be due to a reduction in both the number and sensitivity of cardiac β-receptors; the age-related changes described are akin to the effects of pharmacological β-blockade.
Arterial baroreceptors responses are slowed with increasing age. As a result, aged individuals typically exhibit increased arterial pressure variability with decreased cardiac heart rate variability and responsiveness.21 It has been postulated that these changes predispose to postural hypotension and syncope in the elderly.
It could be argued that discussion of intrinsic ageing and the disease-free heart is a somewhat academic exercise. The reality is that most older people, at least in the developed world, have clinically relevant ischaemic cardiac disease. Atherosclerosis begins in adolescence and the incidence and prevalence of ischaemic heart disease increase dramatically with age.22 Up to 30% of the over 65s have symptoms of ischaemic heart disease with angina or previous acute myocardial infarction. However, it has been estimated that a further 30% have clinically significant but covert or unrecognized disease. A variety of factors contribute to this hidden burden of disease. There may be barriers to communication or reporting such as chronic cognitive impairment. Symptoms may be masked by comorbidities that reduce exercise capacity, or cause other more clinically obvious symptoms. In addition ageing is associated with impaired perception of ischaemic cardiac pain; on exercise there is a delay from onset of myocardial ischaemia to symptoms of angina in older people. As a result when cardiac symptoms occur they may be vague or atypical.23 Myocardial infarction may present as a confusional state or ‘collapse’, while myocardial ischaemia may provoke non-specific lethargy and reduced physical capacity. Therefore, ischaemic heart disease is a common cause of reduced cardiac homeostatic reserve in older people and in the event of a physiological stressor such as systemic illness, adverse clinical consequences such as myocardial ischaemia, heart failure or cardiac arrhythmias can readily occur. The presentation, diagnosis, and treatment of coronary artery disease in older people are discussed in detail in Chapter 37, Ischaemic heart disease.
Cardiac Manifestations of Non-Cardiac Disease
The Brain
Cerebrovascular Disease
Acute strokes in older people, including infarction, subarachnoid and intracranial haemorrhage are often associated with cardiac rhythm or conduction disturbances, and the 12-lead ECG may show repolarization patterns resembling acute myocardial infarction. Elevation in serum cardiac enzymes, particularly troponin, is seen in approximately 20% of patients with acute stroke and is associated with ECG changes and increased mortality. Although some patients will have underlying coronary thrombosis, these ECG and biomarker changes can occur in the absence of significant coronary arterial disease.24 In this situation the underlying process may be a stress response with activation of the sympathoadrenal system causing patchy focal myocardial damage known as myocytolysis.25
Many older subjects with ischaemic cerebrovascular disease have comorbid coronary artery disease. Cardiac disease is the commonest cause of death in the first months following ischaemic stroke, with 2–6% of patients dying from cardiac causes. Risk is highest in the first days immediately post-ictus. Predictors include diabetes, LV dysfunction, renal impairment, long QTc and severe neurological impairment.26
Dementia and Cognitive Decline
Increasingly it is recognized that vascular disease is an important and potentially preventable contributor to dementia and cognitive decline in later life. Post-mortem studies have shown that Alzheimer’s pathology on its own is often not sufficient to cause major cognitive decline. However, the combination of Alzheimer’s pathology and ischaemic cerebrovascular disease (particularly small vessel ischaemia) carries particular risk of dementia.27 Longitudinal population studies have shown that vascular risk factors, particularly hypertension, diabetes mellitus and cigarette smoking, are associated with increased risk of dementia.28, 29 Congestive cardiac failure (CCF) may also contribute to late-life cognitive impairment. Dementia is over-represented in CCF patients; predictors include left ventricular systolic dysfunction and arterial blood pressures below 130 mmHg.30 A variety of mechanisms have been proposed including occult cerebral infarction, low-grade small vessel ischaemia, activated thrombosis and blood pressure variability and altered cerebrovascular reactivity. A degree of cognitive dysfunction occurs in most older patients after cardiac surgery including coronary artery bypass grafting.31 Those with pre-existing small vessel cerebral ischaemia are at particular risk. Low blood pressure during surgery and small cerebral embolic events are thought to be the main contributors.
The Respiratory System
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree